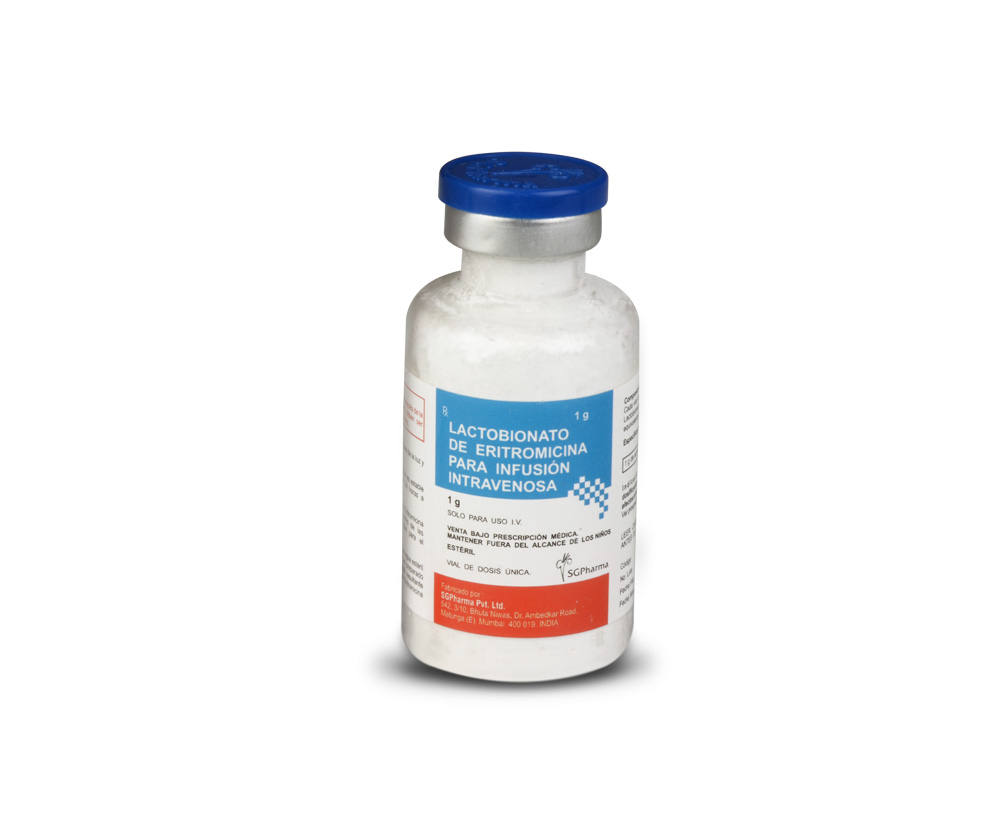
1 gm
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
ERYTHROMYCIN LACTOBIONATE FOR INTRAVENOUS INFUSION B.P. (Erythromycin Lactobionate) is a soluble salt of erythromycin (macrolide antibacterial) suitable for intravenous administration. Chemically, Erythromycin lactobionate is (3R,4S,5S,6R,7R,9R,11R,12R,13S,14R)-4-[(2,6-dideoxy-3-C-methyl-3-O-methyl-α-L-ribo-hexopyranosyl)oxy]-14-ethyl-7,12,13-trihydroxy-3,5,7,9,11,13-hexamethyl-6-[[3,4,6-trideoxy-3-(dimethylamino)-ß-D-xylo-hexopyranosyl]oxy]oxacyclotetradecane-2,10-dione4-O-ß-D-galactopyranosyl-D-gluconate (erythromycin A lactobionate). The molecular formula is C37H67NO13, C12H22O12 and molecular weight is 1092.23.
STRUCTURAL FORMULA :
Its structural formula is :
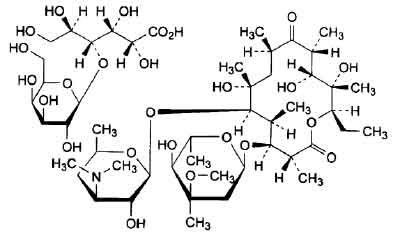
ERYTHROMYCIN LACTOBIONATE FOR INTRAVENOUS INFUSION B.P. is a white to slightly yellow powder filled in clear, flint tubular glass vial of suitable size.
COMPOSITION :
Each vial contains :
Sterile Erythromycin Lactobionate B.P.
equivalent to Erythromycin 1 gm
rythromycin activity 1 gm per vial.
PHARMACOLOGY :
Erythromycin exerts its antimicrobial action by binding to the 50S ribosomal sub-unit of susceptible microorganisms and suppresses protein synthesis. Erythromycin is usually active against most strains of the following organisms both in vitro and in clinical infections :
Gram Positive Bacteria : Listeria monocytogenes, Corynebacterium diphtheriae (as an adjunct to antitoxin), Staphylococci spp, Streptococci spp (including Enterococci).
Gram Negative Bacteria : Haemophilus influenzae, Neisseria meningitidis, Neisseria gonorrhoeae, Legionella pneumophila, Moraxella (Branhamella) catarrhalis, Bordetella pertussis, Campylobacter spp.
Mycoplasma : Mycoplasma pneumoniae, Ureaplasma urealyticum
Other organisms :
Treponema pallidum, Chlamydia spp, Clostridia spp, L-forms, the agents causing trachoma and lymphogranuloma venereum.
Note : The majority of strains of Haemophilus influenzae are susceptible to the concentrations reached after ordinary doses.
PHARMACOKINETICS :
Intravenous infusion of 500 mg of erythromycin lactobionate at a constant rate over 1 hour in fasting adults produced a mean serum erythromycin level of approximately 7 µg/ml at 20 minutes, 10 µg/ml at 1 hour, 2.6 µg/ml at 2.5 hours and 1 µg/ml at 6 hours. The extent of plasma protein binding has been variably reported but is probably of the order of 75 %. Erythromycin diffuses readily into most body fluids with the exception of cerebrospinal fluid, synovial fluid and vitreous humor. In the presence of normal renal function, the plasma half life is approximately1.4 hours; this may increase to 6 hours in anuric patients but does not usually require dosage adjustment. Erythromycin is not removed by dialysis. In the presence of normal hepatic function, erythromycin is concentrated in the liver and is excreted in the bile. However, only small proportion of the administered dose appears in the bile. The effect of hepatic dysfunction on biliary excretion of erythromycin is not known. Approximately 12 to 15 % of an intravenously administered dose of erythromycin is excreted in the urine unchanged. A substantial proportion of the administered dose remains unaccounted for and is presumably metabolized probably in the liver.Erythromycin appears in breast milk at levels which are approximately 50 % of the plasma concentration. It crosses the placenta and foetal plasma levels are usually 5 % - 20 % of the maternal plasma concentration.
Susceptibility Testing :
Dilution or diffusion techniques – either quantitative (MIC) or breakpoint, should be used following a regular updated, recognized and standardized method (eg. CLSI) Standardized susceptibility test procedures require the use of laboratory control microorganisms to control the technical aspects of the laboratory procedures.
- A report of “Susceptible” indicates that the pathogen is likely to be inhibited if the antimicrobial compound in the blood reaches the concentrations usually achievable.
- A report of “Intermediate” indicates that the result should be considered equivocal, and if the microorganism is not fully susceptible to alternative clinically feasible drugs, the test should be repeated. This category implies possible clinical applicability in body sites where the drug is physiologically concentrated or in situations where high dosage of drug can be used. This category also provides a buffer zone, which prevents small – uncontrolled technical factors from causing major discrepancies in interpretation.
- A report of “Resistant” indicates that the pathogen is not likely to be inhibited if the antimicrobial compound in the blood reaches the concentrations usually achievable : other therapy should be selected.
Note 1 : The prevalence of resistance may vary geographically for selected species and local information on resistance is desirable, particularly when treating severe infections.
Note 2 : Many strains of Haemophilus influenzae are resistant to erythromycin alone, but are susceptible to erythromycin and sulfonamides together. Staphylococci resistant to erythromycin may emerge during a course of erythromycin therapy. Culture and susceptibility testing should be performed.
INDICATIONS :
ERYTHROMYCIN LACTOBIONATE FOR INTRAVENOUS INFUSION B.P. is indicated in severe and immunocompromised cases of infections caused by sensitive organisms where high blood levels are required at the earliest opportunity or when the oral route is compromised.
1. Upper respiratory tract infections : Tonsillitis, Peritonsillar abscess, Pharyngitis, Laryngitis, Sinusitis, Secondary infections in influenza and common colds.
2. Lower respiratory tract infections : Tracheitis, acute and chronic bronchitis, Pneumonia (lobar pneumonia, Bronchopneumonia, Primary atypical pneumonia), Bronchiectasis, Legionnaire’s disease.
3. Ear infection : Otitis media and otitis Externa, Mastoiditis.
4. Oral infections : Gingivitis, Vincent’s angina.
5. Eye infections : Blepharitis.
6. Skin and soft tissue infections : Boils and carbuncles, Paronychia, Abscesses, Pustular acne, Impetigo, Cellulitis, Erysipelas.
7. Gastrointestinal infections : Cholecystitis, Staphylococcal enterocolitis.
8. Prophylaxis : Peri-operative secondary infection prophylaxis, Severe trauma and burns Secondary infection prophylaxis, Endocarditis prophylaxis (dental procedures).
9. Septicaemia
10. Endocarditis
11. Other infections : Osteomyelitis, Urethritis, Gonorrhoea, Syphilis, Lymphogranuloma Venereum, Diphtheria, Prostatitis, Scarlet fever.
Administration :
ERYTHROMYCIN LACTOBIONATE FOR INTRAVENOUS INFUSION B.P. is for intravenous (I.V.) infusion use only.
Bolus Injection (I.V. push) is contraindicated.
Continuous infusion with an erythromycin lactobionate is preferred due to the slower infusion rate and lower concentration of erythromycin; however, intermittent infusion at intervals not greater than every six hours is also effective. Intravenous erythromycin should be replaced by oral erythromycin as soon as possible.
Preparations for administration :
For Intermittent Infusion of 1 gram dose :
Step 1 : Add 20 ml of Water for Injections B.P. to the 1 gm vial.
Step 2 : Add 20 ml of Step 1 solution to 200 - 250 ml of Sodium Chloride Intravenous Infusion B.P. (0.9 % Saline). This provides a 0.5 % - 0.4 % solution.
If it is decided to administer the daily dose as an intermittent infusion, then the erythromycin concentration should not exceed 5 mg/ml and the time of each infusion should be between 20 and 60 minutes.
For Continuous Infusion of 1 gram dose :
Step 1 : Add 20 ml of Water for Injections B.P. to the 1 gm vial.
Step 2 : Add 20 ml of Step 1 solution to 500 - 1000 ml of Sodium Chloride Intravenous Infusion B.P. (0.9 % Saline). This provides a 0.2 % - 0.1 % infusion.
The infusion should be completed within eight hours of preparation to ensure potency.
Alternative Step 2 diluents :
Compound Sodium Lactate Injection B.P. (Hartmann’s Solution).
Solutions containing glucose may also be used but sodium bicarbonate must first be added as a buffer to ensure neutrality.
5 ml of sterile 8.4 % w/v sodium bicarbonate solution will neutralize 1 liter of : Glucose Injection B.P. (5 %), or of Sodium Chloride and Glucose Injection B.P. (usually 0.18 % sodium chloride and 4.0 % glucose). The stability of solutions of ERYTHROMYCIN LACTOBIONATE FOR INTRAVENOUS INFUSION B.P. is adversely affected below pH 5.5. Continuous intravenous infusion with an erythromycin concentration of 1 mg/ml (0.1 % solution) is recommended. The infusion should be completed within 8 hours of preparation to ensure potency. If required, solution strengths up to 5 mg/ml (0.5 % solution) may be used, but should not be exceeded. Higher concentrations may result in pain along the vein.
Dosage :
For treatment of acute pelvic inflammatory disease caused by N. Gonorrhoeae, in female patients hypersensitive to penicillins, administer 500 mg erythromycin lactobionate every six hours for three days, followed by oral administration of 250 mg erythromycin stearate or base every six hours for seven days. For treatment of Legionnaires’ Disease : Although optimal doses have not been established, doses utilized in reported clinical data were 1 to 4 grams daily in divided doses. Administration of doses of ≥ 4 gm/day may increase the risk for the development of erythromycin-induced hearing loss in elderly patients, particularly those with reduced renal or hepatic function. In the treatment of Group A beta-haemolytic streptococcal infections of the upper respiratory tract (e.g., tonsillitis or pharyngitis), the therapeutic dosage of erythromycin should be administered for ten days. The American Heart Association suggests a dosage of 250 mg of erythromycin orally, twice a day in long-term prophylaxis of streptococcal upper respiratory tract infections for the prevention of recurring attacks of rheumatic fever in patients allergic to penicillin and sulfonamides. In prophylaxis against bacterial endocarditis the oral regimen for penicillin allergic patients is erythromycin 1 gm, 1 hour before the procedure followed by 500 mg six hours later.
WARNINGS :
There have been reports of hepatic dysfunction, with or without jaundice occurring in patients receiving oral erythromycin products. Clostridium difficile associated diarrhoea (CDAD) has been reported with the use of nearly all antibacterial agents, including erythromycin, and may range in severity from mild diarrhoea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile. C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhoea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents. If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated. Life-threatening episodes of ventricular tachycardia associated with prolonged QT interval (torsades de pointes) have been reported in some patients after intravenous administration of erythromycin lactobionate. Susceptibility to the development of torsades de pointes arrhythmias, a rare but serious cardiac condition, is related to electrolyte imbalance, hepatic dysfunction, myocardial ischemia, left ventricular dysfunction, idiopathic Q-T prolongation, and concurrent antiarrhythmic therapy. Elderly patients exhibit a greater frequency of decreased hepatic function, cardiac function, and of concomitant disease and other drug therapy, and therefore should be monitored carefully during ERYTHROMYCIN LACTOBIONATE FOR INTRAVENOUS INFUSION B.P. therapy.
Pregnancy : Category B
There was no evidence of teratogenicity or any other adverse effect on reproduction in female rats fed erythromycin base (up to 0.25 % of diet) prior to and during mating, during gestation, and through weaning of two successive litters. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed. Erythromycin has been reported to cross the placental barrier in humans, but foetal plasma levels are generally low.
Labor and Delivery :
The effect of erythromycin on labor and delivery is unknown.
Nursing Mothers :
Erythromycin is excreted in breast milk. Caution should be exercised when erythromycin is administered to a nursing woman.
Paediatric Use :
Studies performed to date have not demonstrated paediatrics – specific problems that would limit the usefulness of erythromycin in children.
Geriatric Use :
Elderly patients, particularly those with reduced renal or hepatic function, may be at increased risk for developing erythromycin-induced hearing loss, when ERYTHROMYCIN LACTOBIONATE FOR INTRAVENOUS INFUSION B.P. doses of 4 grams/day or higher are given. Elderly patients may be more susceptible to the development of torsades de pointes arrhythmias than younger patients. Elderly patients may experience increased effects of oral anticoagulant therapy while undergoing treatment with ERYTHROMYCIN LACTOBIONATE FOR INTRAVENOUS INFUSION B.P.
CARCINOGENESIS, MUTAGENESIS AND IMPAIRMENT OF FERTILITY :
Long-term animal data with erythromycin lactobionate for use in determination of possible carcinogenic effects are not available. However, long-term oral studies in rats with erythromycin ethylsuccinate and erythromycin base did not provide evidence of tumorigenicity. Mutagenicity studies have not been conducted. There was no apparent effect on male or female fertility in rats fed erythromycin (base) at levels up to 0.25 % of diet.
INTERACTIONS AND INCOMPATIBILITIES :
Drug Interactions :
Increases in serum concentrations of the following drugs metabolised by the cytochrome P450 system may occur when administered concurrently with erythromycin : acenocoumarol, alfentanil, astemizole, bromocriptine, carbamazepine, cilostazol, cyclosporin, digoxin, dihydroergotamine, disopyramide, ergotamine, hexobarbitone, methylprednisolone, midazolam, omeprazole, phenytoin, quinidine, rifabutin, sildenafil, tacrolimus, terfenadine, theophylline, triazolam, valproate, vinblastine, and antifungals e.g. fluconazole, ketoconazole and itraconazole. Appropriate monitoring should be undertaken and dosage should be adjusted as necessary. Particular care should be taken with medications known to prolong the QTc interval of the electrocardiogram. Drugs that induce CYP3A4 (such as rifampicin, phenytoin, carbamazepine, phenobarbital, St. John’s Wort) may induce the metabolism of erythromycin. This may lead to sub-therapeutic levels of erythromycin and a decreased effect. The induction decreases gradually during two weeks after discontinued treatment with CYP3A4 inducers. Erythromycin should not be used during and two weeks after treatment with CYP3A4 inducers.
HMG-CoA Reductase Inhibitors :
Erythromycin has been reported to increase concentrations of HMG-CoA reductase inhibitors (e.g. lovastatin and simvastatin). Rare reports of rhabdomyolysis have been reported in patients taking these drugs concomitantly.
Contraceptives :
Some antibiotics may in rare cases decrease the effect of contraceptive pills by interfering with the bacterial hydrolysis of steroid conjugates in the intestine and thereby reabsorption of unconjugated steroid. As a result of this plasma levels of active steroid may decrease.
Antihistamine H1 antagonists :
Care should be taken in the co-administration of erythromycin with H1 antagonists such as terfenadine, astemizole and mizolastine due to the alteration of their metabolism by erythromycin. Erythromycin significantly alters the metabolism of terfenadine, astemizole and pimozide when taken concomitantly. Rare cases of serious, potentially fatal, cardiovascular events including cardiac arrest, torsade de pointes and other ventricular arrhythmias have been observed.
Anti-bacterial Agents :
An in vitro antagonism exists between erythromycin and the bactericidal beta-lactam antibiotics (e.g. penicillin, cephalosporin). Erythromycin antagonises the action of clindamycin, lincomycin and chloramphenicol. The same applies for streptomycin, tetracyclines and colistin.
Protease Inhibitors :
In concomitant administration of erythromycin and protease inhibitors, an inhibition of the decomposition of erythromycin has been observed.
Oral Anticoagulants :
There have been reports of increased anticoagulant effects when erythromycin and oral anticoagulants (e.g. warfarin) are used concomitantly.
Triazolobenzodiazepines (such as triazolam and alprazolam) and related benzodiazepines :
Erythromycin has been reported to decrease the clearance of triazolam, midazolam, and related benzodiazepines, and thus may increase the pharmacological effect of these benzodiazepines. Post - marketing reports indicate that co-administration of erythromycin with ergotamine or dihydroergotamine has been associated with acute ergot toxicity characterized by vasospasm and ischaemia of the central nervous system, extremities and other tissues. Elevated cisapride levels have been reported in patients receiving erythromycin and cisapride concomitantly. This may result in QTc prolongation and cardiac arrhythmias including ventricular tachycardia, ventricular fibrillation and torsades de pointes. Similar effects have been observed with concomitant administration of pimozide and clarithromycin, another macrolide antibiotic.
Erythromycin use in patients who are receiving high doses of theophylline may be associated with an increase in serum theophylline levels and potential theophylline toxicity. In case of theophylline toxicity and/or elevated serum theophylline levels, the dose of theophylline should be reduced while the patient is receiving concomitant erythromycin therapy. There have been published reports suggesting when oral erythromycin is given concurrently with theophylline there is a significant decrease in erythromycin serum concentrations. This decrease could result in sub-therapeutic concentrations of erythromycin. There have been post-marketing reports of colchicine toxicity with concomitant use of erythromycin and colchicine. Hypotension, bradyarrhythmias and lactic acidosis have been observed in patients receiving concurrent verapamil, a calcium channel blocker. Cimetidine may inhibit the metabolism of erythromycin which may lead to an increased plasma concentration. Erythromycin has been reported to decrease the clearance of zopiclone and thus may increase the pharmacodynamic effects of this drug.
Incompatibilities :
None stated.
SIDE EFFECTS :
Blood and lymphatic system disorders : Eosinophilia.
Cardiac disorders : QTc interval prolongation, Torsades de pointes, Palpitations, and Cardiac rhythm disorders including Ventricular tachyarrhythmias.
Ear and labyrinth disorders : Deafness, Tinnitus.
There have been isolated reports of reversible hearing loss occurring chiefly in patients with renal insufficiency or taking high doses.
Gastrointestinal disorders :
The most frequent side effects of oral erythromycin preparations are gastrointestinal and are dose-related. The following have been reported : Upper abdominal discomfort, Nausea, Vomiting, Diarrhoea, Pancreatitis, Anorexia, Infantile hypertrophic pyloric stenosis.
Pseudomembranous colitis has been reported rarely in association with erythromycin therapy.
General disorders and administration site conditions : Chest pain, Fever, Malaise.
Hepatobiliary disorders : Cholestatic hepatitis, Jaundice, Hepatic dysfunction, Hepatomegaly, Hepatic failure, Hepatocellular hepatitis.
Immune system disorders : Allergic reactions ranging from urticaria and mild skin eruptions to anaphylaxis have occurred.
Investigations : Increased liver enzyme values.
INFORMATION FOR PATIENTS :
Patients should be counseled that antibacterial drugs including erythromycin should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When erythromycin is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may
(1) Decrease the effectiveness of the immediate treatment and
(2) Increase the likelihood that bacteria will develop resistance and will not be treatable by erythromycin or other antibacterial drugs in the future.
Diarrhoea is a common problem caused by antibiotics which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic. If this occurs, patients should contact their physician as soon as possible.
OVERDOSAGE AND TREATMENT OF OVERDOSAGE :
Symptoms : Hearing loss, severe nausea, vomiting and diarrhoea.
Treatment : In the case of overdosage, ERYTHROMYCIN LACTOBIONATE FOR INTRAVENOUS INFUSION B.P. should be discontinued and all other appropriate measures should be instituted.
Erythromycin is not removed by peritoneal dialysis or haemodialysis.
PHARMACEUTICAL PRECAUTIONS :
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
STORAGE :
Store below 25°C (77°F), protected from moisture and light.
Do not refrigerate.
SHELF LIFE :
24 months from the date of manufacture.
PRESENTATION :
ERYTHROMYCIN LACTOBIONATE FOR INTRAVENOUS INFUSION B.P. contains Erythromycin Lactobionate B.P. equivalent to Erythromycin
1 gm supplied in clear flint tubular glass vial of suitable size.
Single vial per carton.
Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.

 Cardiovascular
Cardiovascular