5 mg/2 ml
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
Verasol (Verapamil HCl) is a slow channel inhibitor or calcium antagonist. Chemically, verapamil hydrochloride is Benzeneacetonitrile, α-[3-[[2-(3,4-dimethoxyphenyl)ethyl] methylamino]propyl]-3,4-dimethoxy-α-(1-methylethyl)-,monohydrochloride,(±)-.The molecular formula is C27H38N2O4.HCl and molecular weight is 491.07.
STRUCTURAL FORMULA :
Its structural formula is :
-Structure.jpg)
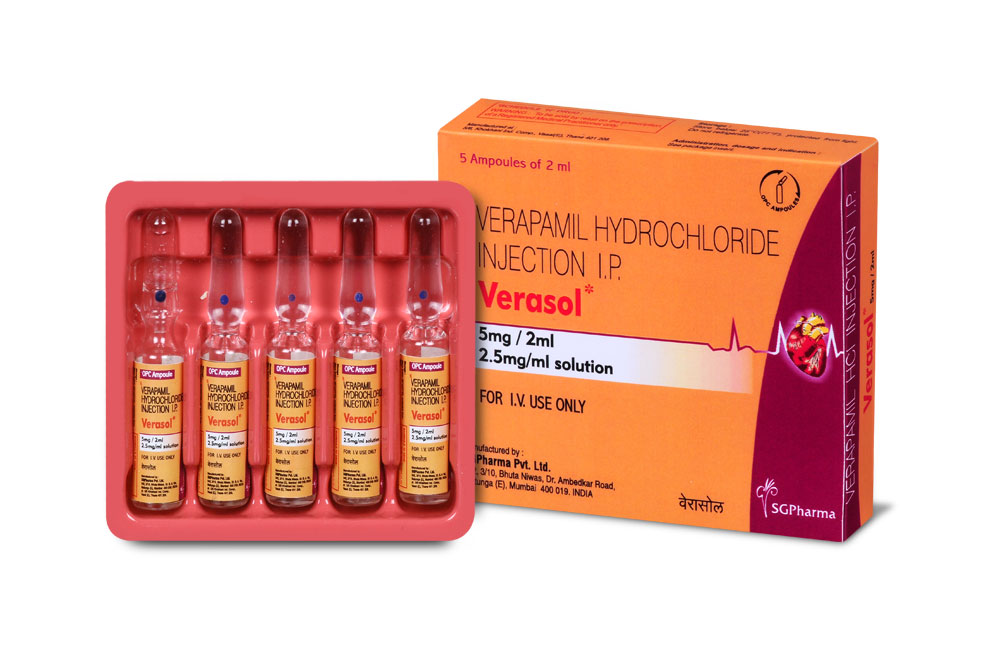
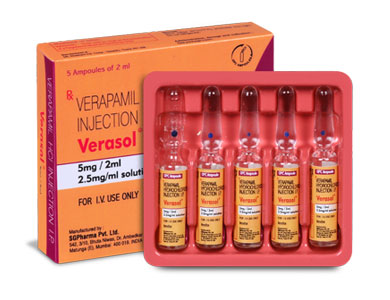
Verasol is a sterile, nonpyrogenic aqueous solution filled in amber glass ampoule of suitable size.
COMPOSITION :
Each ml contains :
Verapamil Hydrochloride USP 2.5 mg
Water for Injection USP q.s.
Contain no preservative.
ACTIONS :
Verapamil has a pronounced antiarrhythmic action particularly in supraventricular cardiac arrhythmias. It prolongs impulse conduction in the AV node and thereby depending on the type of arrhythmia restores the sinus rhythm and/or normalises the ventricular rate. The calcium antagonist verapamil reduces myocardial oxygen consumption directly by intervening in the energy consuming metabolic processes of the myocardial cell and indirectly by diminishing the peripheral resistance (afterload). The decrease of the vascular smooth muscle tone moreover prevents coronary spasms and lowers raised blood pressure.
PHARMACOKINETICS :
Following intravenous infusion in man, verapamil is eliminated bi-exponentially with a rapid distribution phase (half-life about 4 minutes) and a slower terminal elimination phase (half-life 2-5 hours).
INDICATIONS :
- Tachycardias, such as paroxysmal supraventricular tachycardia, atrial fibrillation with rapid ventricular response (except in WPW syndrome), atrial flutter with rapid conduction, extrasystoles.
- For the prophylaxis and/or therapy of ectopic arrhythmias (predominantly ventricular extrasystoles) in halothane anaesthesia and in the application of adrenaline in halothane anaesthesia, respectively.
- Acute hypertension.
- Acute coronary insufficiency.
Administration :
By slow intravenous injection.
INSTRUCTIONS FOR USE OF AMPOULE :
The ampoule used in this product is equipped with O.P.C (One Point Cut) opening system. No ampoule file is needed to open the ampoule. The neck of the ampoule is prescored at the point of constriction. A coloured dot on the ampoule head helps to orientate the ampoule. Take the ampoule and face the coloured dot. Let the solution at the head of the ampoule to flow down by shaking or a gentle stroke. The ampoule opens easily by placing the thumb on the coloured dot and gently pressing downwards as shown.

Dosage :
Adults :
5 mg slowly intravenously, in tachycardias and hypertensive crises repeated, if necessary, after 5 to 10 minutes. Drip infusion to maintain the therapeutic effect : 5-10 mg/hour in physiological saline, glucose, laevulose or similar solutions, on average up to a total dose of 100 mg/day.
Children :
Newborn 0.75 - 1 mg (= 0.3 - 0.4 ml)
Infants 0.75 - 2 mg (= 0.3 - 0.8 ml)
Children
(aged 1 - 5 years) 2 - 3 mg (= 0.8 - 1.2 ml)
(aged 6 - 14 years) 2.5 - 5 mg (= 1 - 2 ml) of Verasol given intravenously, depending on age and action. The injection should be made slowly under electrocardiographic control and only until onset of the effect. Intravenous infusion in hypertensive crises; initially 0.05 - 0.1 mg/kg/hour; if the effect proves to be insufficient, the dose is increased at 30 - 60 minute intervals until twice the dose or more is reached. Average total dose up to 1.5 mg/kg/day.
CONTRAINDICATIONS :
- Cardiogenic shock (except for arrhythmia induced shock), complicated acute myocardial infarction (bradycardia, hypotension, left ventricular failure), second and third degree AV block, sick sinus syndrome (bradycardia-tachycardia syndrome), manifest heart failure.
- In the presence of first degree AV block, sinus bradycardia and hypotension the use of Verasol should be given critical consideration. In acute coronary insufficiency intravenous administration is only admissible with careful indication and continuous monitoring of the patient. Where heart failure is present, full compensation with cardiac glycosides must be achieved before the administration of Verasol.
- Patients with atrial fibrillation or atrial fibrillation and an accessory bypass tract (e.g. Wolff-Parkinson-White, Lown-Ganong-Levine syndromes). These patients are at risk to develop ventricular tachyarrhythmia including ventricular fibrillation if verapamil is administered.
- Patients with ventricular tachycardia. Administration of intravenous verapamil to patients with wide-complex ventricular tachycardia (QRS ≥ 0.12 sec) can result in marked haemodynamic deterioration and ventricular fibrillation. Proper diagnosis and differentiation from wide-complex surpraventricular tachycardia is imperative in the emergency room setting.
- Verasol injection should not be administered intravenously to patients on beta-blockers (except in an intensive care setting).
- In patients with diminished hepatic function (parenchymal loss/reduced blood supply) the effect of Verasol is intensified and prolonged depending on the severity of the disease due to impaired drug metabolism. In these cases, dosage should be adjusted with special care.
- Known hypersensitivity to verapamil hydrochloride.
WARNINGS :
Verapamil may affect impulse conduction. For this reason, Verasol should be used with caution in patients with first degree AV block. Verapamil may affect left ventricular contractility; this effect is small and normally not important but cardiac failure may be precipitated or aggravated. In patients with poor ventricular function, therefore, Verasol should only be given after cardiac failure has been controlled with appropriate therapy, e.g. digitalis.
PRECAUTIONS :
Verapamil should be given as a slow intravenous injection over at least 2 minutes under continuous ECG and blood pressure monitoring. Intravenous injection should only be given by the physician. In atrial fibrillation and simultaneous WPW syndrome there is a risk of inducing ventricular fibrillation.
Hypotension :
Severe hypotension has occasionally occurred following intravenous administration of the drug. On rare occasions this has been followed by a loss of consciousness. If severe hypotension develops, verapamil should be promptly discontinued and vasoconstrictor substances used. In patients using antihypertensive drugs, the additional hypotensive effect should be taken into consideration.
Ventricular Fibrillation :
Intravenous administration may precipitate ventricular fibrillation. Patients with atrial flutter/fibrillation and an accessory AV pathway may develop increased antegrade conduction across the aberrant pathway bypassing the AV node, producing a very rapid ventricular response after receiving intravenous verapamil. Its use in these patients is contraindicated.
Bradycardia/Asystole :
Verasol slows conduction across the AV node and rarely may produce second or third degree AV block, bradycardia and in extreme cases, asystole. This is more likely to occur in patients with a sick sinus syndrome (SA nodal disease). Asystole in patients other than those with sick sinus syndrome is usually of short duration (a few seconds or less), with spontaneous return to AV nodal or normal sinus rhythm. If this does not occur promptly, appropriate treatment should be initiated immediately.
Heart Failure :
Because of the drug’s negative inotropic effect, verapamil should not be used in patients with poorly compensated congestive heart failure, unless the failure is complicated by or caused by an arrhythmia. If verapamil is used in such patients, they must be digitalized prior to treatment. Continuous monitoring is mandatory when intravenous verapamil is used in digitalized patients. It has been reported that digoxin plasma levels may increase with chronic oral administration.
Impaired Hepatic or Renal Function :
Verapamil should be used with caution in patients with hepatic and/or renal impairment. These patients should be monitored carefully for abnormal prolongation of the PR interval or other signs of excessive pharmacological effects.
Progressive Muscular Dystrophy :
Intravenous verapamil can precipitate respiratory muscle failure in these patients and should therefore be used with caution.
Increased Intracranial Pressure :
Intravenous verapamil has been seen to increase intracranial pressure in patients with supratentorial tumors at the time of anaesthesia induction. Caution should be taken and appropriate monitoring performed.
Sick Sinus Syndrome :
Precaution should be taken when treating any supraventricular arrhythmia on an emergency basis as it may be caused by an undiagnosed Sick Sinus Syndrome.
Heart Block :
Development of second or third degree AV block or unifascicular, bifascicular or trifascicular bundle branch block requires reduction in subsequent doses or discontinuation of verapamil and institution of appropriate therapy, if needed.
Pregnancy : Category C
Verapamil carries the potential to produce foetal hypoxia associated with maternal hypotension. Verapamil should not be administered intravenously during the first six months of pregnancy. There are no data on use in the first and second trimester. Verapamil should not be used in the final trimester unless the benefits clearly outweigh the risks.
Nursing mothers :
Verapamil should not be administered intravenously during lactation. If a nursing mother requires intravenous verapamil, breastfeeding should be discontinued for the duration of treatment.
Paediatric Use :
There have been rare cases of severe haemodynamic events - some fatal - after intravenous administration of verapamil to neonates and infants. Intravenous verapamil should not be administered to this group of patients unless it is absolutely necessary and there is no alternative.
INTERACTIONS :
Verapamil has been shown to increase the serum concentration of digoxin and caution should be exercised with regard to digitalis toxicity. The digitalis level should be determined and the glycoside dose reduced, if required. The combination of Verasol and beta-blockers, anti-arrhythmic agents or inhaled anaesthetics may lead to additive cardiovascular effects (e.g. AV block, bradycardia, hypotension, heart failure). Verasol should not be given in combination with intravenous beta-blocker therapy and care must be exercised if Verasol is combined with oral beta-blocker therapy or anti-arrhythmic agents by any route. The effects of Verasol may be additive to other hypotensive agents. Interactions between verapamil and the following have been reported :
SIDE EFFECTS :
As with all drugs which inhibit AV conduction, Verasol can produce first or second degree AV block; in extreme cases there may be complete block with or without subsequent asystole. Occasionally, heart failure may develop or existing heart failure may be exacerbated.
The risk of inducing ventricular fibrillation is minute, as Verasol has no effect on the conduction velocity and refractory period in either atria or ventricles. By diminishing the peripheral resistance, the intravenous administration of Verasol may lead to a slight and transient decrease of blood pressure even in normotensive patients. If the heart is no longer able to increase cardiac output for maintaining normal blood pressure, a critical blood pressure fall may occur. There are rare reports of symptoms such as palpitations and rapid heat beat (tachycardia) in patients receiving verapamil. Elevation of the pacing and sensing threshold cannot be ruled out in pacemaker wearers on verapamil hydrochloride. Frequently, nausea (rarely, vomiting), bloating or constipation - in isolated cases to the point of ileus, abdominal discomfort and pain. Occasionally, there may be headache, nervousness, dizziness or lightheadedness, fatigue, sensory disturbances such as tingling, numbness (paraesthesia, neuropathy), shakiness (tremor) and vertigo.
Flush has been observed occasionally. Occasionally, allergic reactions such as erythema, pruritus, urticaria, maculopapular exanthema and erythromelalgia may occur. Rarely bronchospasm may occur. Rarely - tinnitus. Peripheral oedema may occur as a result of local arteriole dilation. Rarely, reversible elevation of liver enzymes has been observed, probably as a manifestation of allergic hepatitis. Relevant lowering of glucose tolerance is rare. There are rare reports of impotence. Gynaecomastia has been observed very rarely in elderly patients on long term treatment. In the cases reported to date, the condition was reversible upon discontinuation of the drug. Elevated prolactin levels have been described, with isolated cases of milk (galactorrhoea). Very rarely, there have been cases of purpura in the skin or mucous. There are isolated reports of photodermatitis. Very rarely, muscular weakness or muscle and joint pain may occur. There are isolated reports of angioneurotic oedema and Stevens-Johnson syndrome.There may be isolated cases of gingival hyperplasia which is reversible when the drug is discontinued.
OVERDOSAGE :
Symptoms :
Hypotension, bradycardia up to high degree AV block and sinus arrest, hyperglycemia, stupor and metabolic acidosis. Fatalities have occurred as a result of overdose.
TREATMENT OF OVERDOSAGE :
Treatment of overdosage should be supportive and individualized. Beta-adrenergic stimulation and/or parenteral administration of calcium injection (calcium chloride) have been effectively used in treatment of deliberate overdosage with oral verapamil hydrochloride. Verapamil hydrochloride cannot be removed by hemodialysis. Clinically significant hypotensive reactions or high-degree AV block should be treated with vasopressor agents or cardiac pacing, respectively. Asystole should be handled by the usual measures including isoproterenol hydrochloride, other vasopressor agents or cardiopulmonary resuscitation.
PHARMACEUTICAL PRECAUTIONS :
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
STORAGE :
Store below 30°C (86°F), protected from light.
Do not refrigerate.
SHELF LIFE :
24 months from the date of manufacture.
PRESENTATION :
Verasol is supplied as 5 mg Verapamil Hydrochloride in 2 ml aqueous solution.
Such 5 ampoules of 2 ml are packed in a Box.
Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.

 Cardiovascular
Cardiovascular