500 mg/5 ml
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
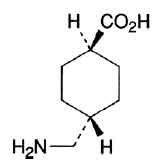
TRANEXAMIC ACID INJECTION (Tranexamic Acid) is a synthetic derivative of the amino acid lysine that exerts its antifibrinolytic effect by blocking lysine-binding sites on plasminogen molecules and thereby inhibiting the interaction of plasminogen and the heavy chain of plasmin with lysine residues on the surface of fibrin. Although plasmin can still be formed under these circumstances, it is unable to bind to and degrade fibrin. Tranexamic acid is 6 to 10 times more potent in terms of binding to plasminogen/plasmin than the other synthetic antifibrinolytic agent E-aminocaproic acid (EACA). Chemically, tranexamic acid is trans-4-(Aminomethyl)cyclohexanecarboxylic acid. The molecular formula is C8H15NO2 and molecular weight is 157.2.
STRUCTURAL FORMULA :
Its structural formula is :
TRANEXAMIC ACID INJECTION is clear colourless sterile solution filled in 5 ml amber glass ampoule.
COMPOSITION :
Each ml contains :
Tranexamic Acid B.P. 100 mg
Water for Injections I.P. q.s.
Contains no preservatives.
ACTIONS :
Tranexamic acid is a competitive inhibitor of plasminogen activation and at much higher concentrations a noncompetitive inhibitor of plasmin, thus implying that tranexamic acid interferes with the fibrinolytic process in the same way as aminocaproic acid. Tranexamic acid is about 10 times more potent in vitro than aminocaproic acid. Tranexamic acid binds considerably more strongly than aminocaproic acid to both the strong and weak sites of the plasminogen molecule in a ratio corresponding to the difference in potency between the compounds. Tranexamic acid in a concentration of 1 mg/ml does not aggregate platelets in vitro. Tranexamic acid in concentrations up to 10 mg/ml blood has no influence on the platelet count, the coagulation time or various coagulation factors in whole blood or citrated blood in normal subjects. On the other hand tranexamic acid in concentrations of 10 mg/ml and 1 mg/ml blood prolongs the thrombin time. Tranexamic acid does not bind to serum albumin. The plasma protein binding is about 3 % at therapeutic plasma levels and seems to be fully accounted for by its binding to plasminogen.
PHARMACOKINETICS :
Three hours after a single oral dose of 25 mg/kg, the peak serum level was 15.4 g/l and the aqueous humour level was 1.6 g/l. The total amount of metabolites excreted in urine during 72 hours is less than 5 %. Possible routes of biotransformation are acetylation or deamination followed by oxidation or reduction. After oral administration approximately 50 % of the parent compound, 2 % of the deaminated dicarboxylic acid and 0.5 % of the acetylated product are excreted. Tranexamic acid is eliminated by glomerular filtration, excretion being about 90 % at 24 hours after intravenous administration of 10 mg/kg bodyweight. After oral administration of 10 to 15 mg/kg body weight the urinary excretion at 24 hours is 39 % and at 48 hours is 41 %. The plasma peak level after 1 g orally is 8 mg/l and after 2 g, 15 mg/l, both obtained three hours after dosing. A parallel intake of food has no influence on the bioavailability of the drug. When administered 36 to 48 hours before surgery in 4 doses of 10 to 20 mg/kg, an antifibrinolytically active concentration (10 μg/ml) of tranexamic acid remains in different tissues for about 17 hours and in the serum for up to seven or eight hours.
Tranexamic acid passes through to the placenta. The concentration in cord blood after an intravenous injection of 10 mg/kg to women could be fairly high, about 30 μg/ml of foetal serum. The concentration in breast milk is about one hundredth of the serum peak concentration obtained. Tranexamic acid passes to semen and inhibits its fibrinolytic activity but does not influence the sperm migration. Tranexamic acid crosses the blood-brain barrier. The drug passes into the aqueous humour, the concentration being about one tenth of the plasma concentration. Tranexamic acid diffuses rapidly to the joint fluid and the synovial membrane, and in the joint fluid the same concentration is obtained as in the serum. The biological half-life in the joint fluid is about three hours.
INDICATIONS :
Local fibrinolysis
For short term use in prophylaxis and treatment in patients at high risk of per - and post-operative haemorrhage following :
a) prostatectomy
b) conisation of the cervix
c) surgical procedures and dental extractions in haemophiliacs
General fibrinolysis
a) Haemorrhagic complications in association with thrombolytic therapy.
b) Haemorrhage associated with disseminated intravascular coagulation with predominant activation of the fibrinolytic system.
Administration :
By slow intravenous injection.
INSTRUCTIONS FOR USE OF AMPOULE :
The ampoule used in this product is equipped with O.P.C (One Point Cut) opening system. No ampoule file is needed to open the ampoule. The neck of the ampoule is prescored at the point of constriction. A coloured dot on the ampoule head helps to orientate the ampoule. Take the ampoule and face the coloured dot. Let the solution at the head of the ampoule to flow down by shaking or a gentle stroke. The ampoule opens easily by placing the thumb on the coloured dot and gently pressing downwards as shown.

Dosage :
Intravenous administration is necessary only if it is difficult to give adequate doses by mouth. The recommended standard dose is 2-3 tablets of 0.5 g, or 5-10 ml by slow intravenous injection at a rate of 1 ml/minute, two to three times daily. For the indications listed below the following doses are recommended.
Prostatectomy :
5-10 ml by slow intravenous injection every eight hours (the first injection being given during the operation) for the first three days after surgery; thereafter 1-1.5 g orally three to four times daily until macroscopic haematuria is no longer present.
Menorrhagia :
1-1.5 g orally three to four times daily for three to four days. tranexamic acid therapy is initiated when bleeding has become profuse.
Epistaxis :
1.5 g orally three times daily for four to ten days. TRANEXAMIC ACID INJECTION may be applied topically to the nasal mucosa of patients suffering from epistaxis. This can be done by soaking a gauze strip in the solution, and then packing the nasal cavity.
Haematuria :
1-1.5 g orally 2 - 3 times daily until macroscopic haematuria is no longer present.
Conisation of the Cervix :
1.5 g orally 3 times a day for 12 to 14 days post-operatively.
Dental Surgery in Patients with Coagulopathies :
Immediately before surgery, 10 mg per kg body-weight should be given intravenously. After surgery, 25 mg per kg body-weight is given orally three to four times daily for six to eight days. Coagulation factor concentrate might be necessary to administer.
General fibrinolysis :
1.0 g (10 ml) by slow intravenous injection three to four times daily. With fibrinolysis in conjunction with diagnosed, increased intravascular coagulation i.e. defibrillation syndrome, an anticoagulant such as heparin may be given with caution.
Hereditary angioneurotic oedema :
1-1.5 g orally two to three times daily as intermittent or continuous treatment depending on whether the patient has prodromal symptoms or not.
Renal insufficiency :
For patients with impaired renal function, the following dosages are recommended :
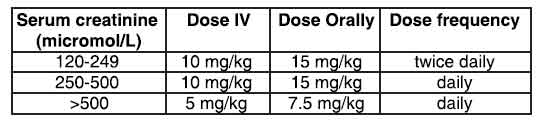
Children :
Clinical experience with TRANEXAMIC ACID INJECTION in menorrhagic children under 15 years of age is not available.
CONTRAINDICATIONS :
TRANEXAMIC ACID INJECTION is contraindicated :
1.In patients with active intravascular clotting process (primary fibrinolysis must be differentiated from disseminated intravascular coagulation)
2.In patients with acquired defective colour vision (used as an indicator of toxicity)
3.In patients with sub-arachnoid haemorrhage (potential occurrence of cerebral ischaemic complications)
4.Hypersensitivity to tranexamic acid
5.Severe renal insufficiency
WARNINGS :
Focal areas of retinal degeneration have developed in cats, dogs and rats following oral or intravenous tranexamic acid at doses between 250 to 1600 mg/kg/day (6 to 40 times the recommended usual human dose) from 6 days to 1 year. The incidence of such lesions has varied from 25 % to 100 % of animals treated and was dose-related. At lower doses some lesions have appeared to be reversible. Limited data in cats and rabbits showed retinal changes in some animals with doses as low as 126 mg/kg/day (only about 3 times the recommended human dose) administered for several days to two weeks. No retinal changes have been reported or noted in eye examinations in patients treated with tranexamic acid for weeks to months in clinical trials. However, visual abnormalities, often poorly characterized, represent the most frequently reported postmarketing adverse reaction in Sweden. For patients who are to be treated continually for longer than several days, an ophthalmological examination, including visual acuity, colour vision, eye-ground and visual fields, is advised, before commencing and at regular intervals during the course of treatment. Tranexamic acid should be discontinued if changes in examination results are found.
PRECAUTIONS :
General
The dose of TRANEXAMIC ACID INJECTION should be reduced in patients with renal insufficiency because of the risk of accumulation. Ureteral obstruction due to clot formation in patients with upper urinary tract bleeding has been reported in patients treated with TRANEXAMIC ACID INJECTION. Venous and arterial thrombosis or thromboembolism has been reported in patients treated with TRANEXAMIC ACID INJECTION. In addition, cases of central retinal artery and central retinal vein obstruction have been reported.
Patients with a previous history of thromboembolic disease may be at increased risk for venous or arterial thrombosis. TRANEXAMIC ACID INJECTION should not be administered concomitantly with Factor IX Complex concentrates or Anti-inhibitor Coagulant concentrates, as the risk of thrombosis may be increased. Patients with disseminated intravascular coagulation (DIC), who require treatment with TRANEXAMIC ACID INJECTION, must be under strict supervision of a physician experienced in treating this disorder.
Pregnancy : Category B
There are no adequate and well-controlled studies in pregnant women. However, tranexamic acid is known to pass the placenta. It appears in cord blood at concentrations approximately equal to maternal concentration. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Nursing mothers :
Tranexamic acid is present in the mother’s milk at a concentration of about a hundredth of the corresponding serum levels. Caution should be exercised when TRANEXAMIC ACID INJECTION is administered to a nursing woman.
Paediatric Use :
The drug has had limited use in paediatric patients, principally in connection with tooth extraction. The limited data suggest that dosing instructions for children can be used for paediatric patients needing tranexamic acid therapy.
Geriatrics :
Appropriate studies performed to date have not demonstrated geriatrics-specific problems that would limit the usefulness of tranexamic acid in the elderly.
INTERACTIONS AND INCOMPATIBILITIES :
Since limited data are available on the co-administration of highly activated prothrombin complex products and antifibrinolytic agents, anti-inhibitor coagulant complex is not to be used concomitantly with tranexamic acid. Tranexamic acid can be mixed with most solutions such as electrolyte, carbohydrate, amino acid, and dextran solutions. It is compatible with heparin. The drug should not be mixed with transfusion blood or with infusion solutions containing penicillin. Concomitant chlorpromazine and tranexamic acid therapy of subarachnoid haemorrhage has been reported to result in cerebral vasospasm and cerebral ischaemia, and possibly a reduction in cerebral blood flow. The sympathomimetic properties of both drugs may have contributed to the development of vasospasm and cerebral ischaemia in these patients. It is best to avoid the combination during treatment of subarachnoid haemorrhage.
SIDE EFFECTS :
Gastrointestinal disturbances (nausea, vomiting, diarrhoea) may occur but disappear when the dosage is reduced. Giddiness and hypotension have been reported occasionally. Hypotension has been observed when IV injection is too rapid. To avoid this response, the solution should not be injected more rapidly than 1 mL per minute. This adverse reaction has not been reported with oral administration.
OVERDOSAGE AND TREATMENT OF OVERDOSAGE :
No cases of overdosage have been reported. Symptoms may be nausea, vomiting, orthostatic symptoms and/or hypotension. Maintain a high fluid intake to promote renal excretion.
PHARMACEUTICAL PRECAUTIONS :
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
STORAGE :
Store below 30°C.
Do not refrigerate.
SHELF LIFE :
24 months from the date of manufacture.
PRESENTATION :
TRANEXAMIC ACID INJECTION is supplied as 500 mg of Tranexamic Acid B.P. in 5 ml Ampoule.
5 Ampoules of 5 ml per box.
Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.

 Cardiovascular
Cardiovascular