100 mg/ml
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
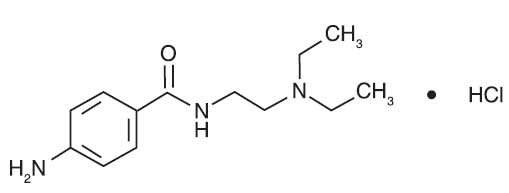
PROCAINAMIDE HYDROCHLORIDE INJECTION USP (Procainamide Hydrochloride) is a Group 1A cardiac antiarrhythmic drug. Chemically, Procainamide Hydrochloride is p-Amino-N-[2-(diethylamino)ethyl]benzamide monohydrochloride. The molecular formula is C13H21N3O ·HCl and molecular weight is 271.79.
STRUCTURAL FORMULA :
Its structural formula is :
PROCAINAMIDE HYDROCHLORIDE INJECTION USP is a clear, colourless or very pale yellow sterile solution filled in 1 ml amber glass ampoule.
COMPOSITION :
Each ml contains :
Procainamide Hydrochloride USP 100 mg
Benzyl Alcohol USP 0.9 % v/v
(as preservative)
Water for Injection USP q.s.
ACTIONS :
Procainamide (PA) increases the effective refractory period of the atria, and to a lesser extent the bundle of His-Purkinje system and ventricles of the heart. It reduces impulse conduction velocity in the atria, His-Purkinje fibers, and ventricular muscle, but has variable effects on the atrioventricular (A-V) node, a direct slowing action and a weaker vagolytic effect which may speed A-V conduction slightly. Myocardial excitability is reduced in the atria, Purkinje fibers, papillary muscles, and ventricles by an increase in the threshold for excitation, combined with inhibition of ectopic pacemaker activity by retardation of the slow phase of diastolic depolarization, thus decreasing automaticity especially in ectopic sites. Contractility of the undamaged heart is usually not affected by therapeutic concentrations, although slight reduction of cardiac output may occur, and may be significant in the presence of myocardial damage. Therapeutic levels of Procainamide may exert vagolytic effects and produce slight acceleration of heart rate, while high or toxic concentrations may prolong A-V conduction time or induce A-V block, or even cause abnormal automaticity and spontaneous firing by unknown mechanisms.
The electrocardiogram may reflect these effects by showing slight sinus tachycardia (due to the anticholinergic action) and widened QRS complexes and, less regularly, prolonged Q-T and P-R intervals (due to longer systole and slower conduction), as well as some decrease in QRS and T wave amplitude. These direct effects of Procainamide on electrical activity, conduction, responsiveness, excitability and automaticity are characteristic of a Group 1A antiarrhythmic agent, the prototype for which is quinidine; Procainamide effects are very similar. However, Procainamide has weaker vagal blocking action than does quinidine, does not induce alpha-adrenergic blockade, and is less depressing to cardiac contractility.
PHARMACOKINETICS :
Following intramuscular injection, procainamide is rapidly absorbed into the bloodstream, and plasma levels peak in 15 to 60 minutes, considerably faster than orally administered procainamide hydrochloride tablets or capsules which produce peak plasma levels in 90 to 120 minutes. Intravenous administration of Procainamide Hydrochloride Injection can produce therapeutic procainamide levels within minutes after infusion is started. About 15 to 20 percent of Procainamide is reversibly bound to plasma proteins, and considerable amounts are more slowly and reversibly bound to tissues of the heart, liver, lung, and kidney. The apparent volume of distribution eventually reaches about 2 liters per kilogram body weight with a half-time of approximately five minutes. While Procainamide has been shown in the dog to cross the blood-brain barrier, it did not concentrate in the brain at levels higher than in plasma. It is not known if Procainamide crosses the placenta. Plasma esterases are far less active in hydrolysis of Procainamide than of procaine. The half-time for elimination of Procainamide is three to four hours in patients with normal renal function, but reduced creatinine clearance and advancing age each prolong the half-time of elimination of Procainamide.
A significant fraction of the circulating Procainamide may be metabolized in hepatocytes to N-acetylprocainamide (NAPA), ranging from 16 to 21 percent of an administered dose in “slow acetylators” to 24 to 33 percent in “fast-acetylators”. Since N-acetylprocainamide also has significant antiarrhythmic activity and somewhat slower renal clearance than Procainamide, both hepatic acetylation rate capability and renal function, as well as age, have significant effects on the effective biologic half-time of therapeutic action of administered Procainamide and the N-acetylprocainamide derivative. Trace amounts may be excreted in the urine as free and conjugated p-aminobenzoic acid, 30 to 60 percent as unchanged Procainamide, and 6 to 52 percent as the N-acetylprocainamide derivative. Both Procainamide and N-acetylprocainamide are eliminated by active tubular secretion as well as by glomerular filtration. Action of Procainamide on the central nervous system is not prominent, but high plasma concentrations may cause tremors. While therapeutic plasma levels for Procainamide have been reported to be 3 to 10 mcg/ml certain patients such as those with sustained ventricular tachycardia, may need higher levels for adequate control. This may justify the increased risk of toxicity. Where programmed ventricular stimulation has been used to evaluate efficacy of Procainamide in preventing recurrent ventricular tachyarrhythmias, higher plasma levels (mean, 13.6 mcg/ml) of Procainamide were found necessary for adequate control.
INDICATIONS :
PROCAINAMIDE HYDROCHLORIDE INJECTION USP is indicated for acute reversion of life-threatening or severely symptomatic ventricular tachyarrhythmias in a hospital, during continuous ECG monitoring. Because of the proarrhythmic effects of PROCAINAMIDE HYDROCHLORIDE INJECTION USP, its use with lesser arrhythmias is not recommended. Class 1 antiarrhythmic drugs have not been shown to enhance survival in patients with ventricular arrhythmias. Because procainamide has the potential to produce serious haematological disorders (0.5 percent) particularly leukopenia or agranulocytosis (sometimes fatal), its use should be reserved for patients in whom, in the opinion of the physician, the benefits of treatment clearly outweigh the risks.
Administration :
PROCAINAMIDE HYDROCHLORIDE INJECTION USP is intended for intravenous or intramuscular administration.
INSTRUCTION FOR USE OF AMPOULE :
The ampoule used in this product is equipped with O.P.C (One Point Cut) opening system. No ampoule file is needed to open the ampoule. The neck of the ampoule is prescored at the point of constriction. A coloured dot on the ampoule head helps to orientate the ampoule. Take the ampoule and face the coloured dot. Let the solution at the head of the ampoule to flow down by shaking or a gentle stroke. The ampoule opens easily by placing the thumb on the coloured dot and gently pressing downwards as shown.

Dosage :
The dosage needed to maintain the therapeutic effect should be assessed principally from the clinical response and will depend upon the patient’s weight and age, renal elimination, hepatic acetylation rate, general condition and cardiac status, but should be adjusted for each patient based upon close observation. Parenteral administration is used in arrhythmias that require immediate suppression. Intravenous therapy allows most rapid control of serious arrhythmias, including those following myocardial infarction; it should be carried out only in settings in which close observation and ECG monitoring of the patient are possible (eg, a hospital or other emergency facility). Intramuscular administration is less likely to produce temporary high plasma levels, but therapeutic plasma levels are not obtained as rapidly as with intravenous administration. Intravenous therapy should be terminated if persistent conduction disturbances or hypotension develop. Parenteral administration is also preferable in patients who are experiencing nausea or vomiting, patients who are to receive nothing by mouth preoperatively, and patients in whom there is reason to believe that absorption may be unreliable. When switching from parenteral to oral therapy, a period of approximately 3 to 4 hours (one half the time for renal elimination, ordinarily) should elapse between the last intravenous dose and the first oral dose.
Renal or Hepatic Impairment
Reduced renal function will prolong the elimination half-life and lower the dose rate needed to maintain therapeutic levels. Hepatic impairment also decreases elimination of procainamide and its metabolites. Reduced doses or longer dosing intervals may produce adequate blood levels and decrease the probability of occurrence of dose-related adverse reactions.
Age/Cardiac Insufficiency
Advancing age reduces the renal excretion of procainamide and N-acetylprocainamide independently of reductions in creatinine clearance. Compared to renal excretion in normal young adults, there is approximately a 25 % reduction at age 50, and a 50 % reduction at age 75. Therefore, in older patients and those with cardiac insufficiency, lower doses or longer dosing intervals may produce adequate blood levels and decrease the probability of occurrence of dose-related adverse reactions.
Intramuscular Initial Dose
An initial daily dose of 50 mg per kg body weight may be estimated. This amount should be divided into fractional doses of one eighth to one quarter to be injected intramuscularly every three to six hours until oral therapy is possible. If more than three injections are given, the physician may wish to assess patient characteristics, clinical response and, if available, blood levels of procainamide and N-acetylprocainamide in adjusting further doses for that individual.
Surgery
For treatment of arrhythmias associated with anaesthesia or surgical operation, the suggested dose is 100 to 500 mg by intramuscular injection.
Intravenous
Intravenous administration of PROCAINAMIDE HYDROCHLORIDE INJECTION USP should be done cautiously to avoid a possible hypotensive response. Initial arrhythmia control, under blood pressure and ECG monitoring, can usually be accomplished safely within a half-hour by either of the two methods which follow :
a) Repeat Bolus Injection
Direct injection into a vein or into tubing of an established infusion line should be done slowly at a dose of 100 mg every 5 minutes and at a rate not to exceed 50 mg per minute. It is advisable to dilute PROCAINAMIDE HYDROCHLORIDE INJECTION USP prior to intravenous injection to facilitate control of dosage rate. Once the arrhythmia is suppressed, or 500 mg has been administered, it is advisable to wait 10 minutes or longer to allow for more distribution into tissues before resuming. The maximum advisable dosage to be given is 1 gm.
b) Loading Injection
Alternatively, a loading infusion containing 20 mg of PROCAINAMIDE HYDROCHLORIDE INJECTION USP per ml (1 gm diluted to 50 ml with 5 % glucose) may be administered at a constant rate of 1 ml per minute for 25 to 30 minutes to deliver 500 to 600 mg of procainamide. Some effects may be seen after infusion of the first 100 or 200 mg; it is unusual to require more than 600 mg to achieve satisfactory antiarrhythmic effects. The maximum advisable dosage to be given is 1 gm.
Maintenance Dose
To maintain therapeutic levels, a more dilute intravenous infusion at a concentration of 2 mg/ml (1 gm PROCAINAMIDE HYDROCHLORIDE INJECTION USP in 500 ml of 5 % glucose), may be administered at 1 to 3 ml/minute. If daily total fluid intake must be limited, a 4 mg/ml concentration (1 gm of PROCAINAMIDE HYDROCHLORIDE INJECTION USP in 250 ml of 5 % glucose) administered at 0.5 to 1.5 ml/minute will deliver an equivalent 2 to 6 mg per minute. A maintenance infusion rate of 50 mcg/min/kg body weight to a person with a normal renal procainamide elimination half-time of three hours may be expected to produce a plasma level of approximately 6.5 mcg/ml. Parenteral drug products should be examined visually for particulate matter and discolouration prior to administration. During intravenous administration of PROCAINAMIDE HYDROCHLORIDE INJECTION USP, blood pressure and ECG should be monitored continuously, and the rate of administration adjusted accordingly. If a fall in blood pressure of more than 15 mmHg occurs, or if excessive widening of the QRS complex (greater than 50 %) or prolongation of the PR interval occurs, or if severe adverse effects appear, the drug should be temporarily discontinued.
Intravenous therapy should be terminated as soon as the patient’s basic cardiac rhythm appears stabilised, and, if indicated, the patient should be placed on oral antiarrhythmic maintenance therapy. When switching from parenteral to oral therapy, a period of approximately 3 to 4 hours (one half the time for renal elimination, ordinarily) should elapse between the last intravenous dose and the first oral dose. The table below provides dosage and infusion rates for both initial loading dose and maintenance infusions
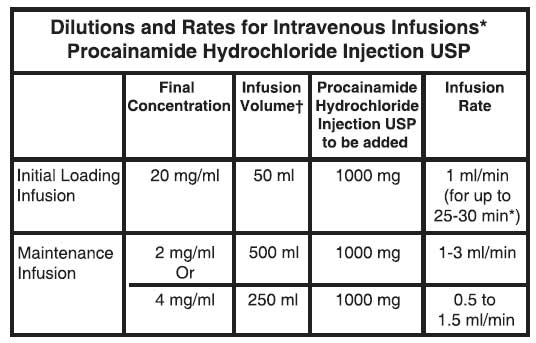
The maintenance infusion rates are calculated to deliver 2 to 6 mg per minute, depending on body weight, renal elimination rate, and steady-state plasma level needed to maintain control of the arrhythmia*. The 4 mg/ml maintenance concentration may be preferred if total infusion volume must be limited. †All infusions should be made up to final volume with 5 % glucose. The flow rate of any intravenous procainamide infusion must be monitored closely to avoid transiently high plasma levels and possible hypotension.
CONTRAINDICATIONS :
Heart Block
Procainamide should not be administered to patients with complete heart block because of its effects in suppressing nodal or ventricular pacemakers and the hazard of asystole. It may be difficult to recognise complete heart block in patients with ventricular tachycardia, but if significant slowing of ventricular rate occurs during procainamide treatment without evidence of A-V conduction appearing, procainamide should be stopped. In cases of second degree A-V block or various types of hemiblock, procainamide should be avoided or discontinued, because of the possibility of increased severity of block, unless the ventricular rate is controlled by an electrical pacemaker.
Lupus Erythematosus
An established diagnosis of systemic lupus erythematosus is a contraindication to procainamide therapy, since aggravation of symptoms is highly likely.
Torsades de Pointes
In torsades de pointes (twistings of the points), characterised by rotation of the axis of the QRS complexes of the ventricular tachycardia in persons with prolonged Q-T and often enhanced U waves, Class 1A antiarrhythmic drugs are contraindicated. Administration of procainamide in such cases may aggravate this special type of ventricular extrasystole or tachycardia instead of suppressing it.
Hypersensitivity
Hypersensitivity to PROCAINAMIDE HYDROCHLORIDE INJECTION USP is an absolute contraindication. In patients sensitive to procaine or other ester-type local anaesthetics, cross sensitivity should be considered; procainamide should not be used if it produces acute allergic dermatitis, asthma or anaphylactic symptoms.
Children
PROCAINAMIDE HYDROCHLORIDE INJECTION USP is not recommended for use in children.
PRECAUTIONS :
Electrolyte Imbalance
Electrolyte imbalance may affect the action of procainamide and the drug should not be used in patients with hypokalaemia. In order to reduce the occurrence of proarrhythmia, potassium levels should be maintained at not less than 4 mmol/l in patients taking procainamide.
Cardiac Effects
During administration of the medicine, evidence of untoward myocardial responses should be carefully watched for in all patients. In the presence of an abnormal myocardium, procainamide may at times produce untoward responses. Correction of atrial fibrillation, with resultant forceful contractions of the atrium, may cause a dislodgement of mural thrombi and produce an embolic episode. Attempts to adjust the heart rate in a patient who has developed ventricular tachycardia during an occlusive coronary episode should be carried out with extreme caution. Caution is also required in marked disturbances of atrioventricular conduction such as A-V block, bundle branch block, or severe digitalis intoxication, where the use of procainamide may result in additional depression of conduction and ventricular asystole or fibrillation.
Blood-Pressure and ECG Monitoring
Blood pressure should be monitored with the patient supine during parenteral, especially intravenous, administration of procainamide. There is a possibility that relatively high although transient plasma levels of procainamide may be attained and cause hypotension before the procainamide can be distributed from the plasma to its full apparent volume of distribution which is approximately 50 times greater. Therefore, caution should be exercised to avoid overly rapid administration of procainamide. If the blood pressure falls 15 mm Hg or more, procainamide administration should be temporarily discontinued. During parenteral administration electrocardiographic (ECG) monitoring is required as well, both for observation of the progress and response of the arrhythmia under treatment, and for early detection of any tendency to excessive widening of the QRS complex, prolongation of the P-R interval, or any signs of heart block. If electrocardiograms give evidence of impending heart block, parenteral administration should be discontinued at once. Since patients with severe organic heart disease and ventricular tachycardia may also have complete heart block which is difficult to diagnose under these circumstances, this complication should always be kept in mind when treating ventricular arrhythmias with procainamide (especially parenterally). If the ventricular rate is significantly slowed by procainamide without attainment of regular atrioventricular conduction, the medicine should be stopped and the patient re-evaluated as asystole may result under these circumstances. Parenteral therapy with procainamide should be limited to use in hospitals in which monitoring and intensive supportive care are available, or to emergency situations in which equivalent observation and treatment can be provided.
Clinical/ECG Monitoring
After achieving and maintaining therapeutic plasma concentrations and satisfactory electrocardiographic and clinical responses, continued frequent periodic monitoring of vital signs and electrocardiograms is advised. If evidence of QRS widening of more than 25 percent or marked prolongation of the Q-T interval occurs, concern for overdosage is appropriate, and interruption of the procainamide infusion is advisable if a 50 percent increase in QRS widening occurs.
Pregnancy : Category C.
Procainamide crosses the placenta. Adequate and well-controlled studies have not been done in humans. Some reports of procainamide use in pregnant women seem to indicate that although procainamide and N-acetylprocainamide (NAPA) appear in foetal serum, no adverse effects on the foetus or neonate have been noted. However, there is a potential risk of drug accumulation and maternal hypotension leading to uteroplacental insufficiency and ventricular arrhythmias.
Nursing Mothers :
Both procainamide and N-acetylprocainamide are excreted in human milk, and absorbed by the breast fed infant. Because of the potential for serious adverse reactions in breast fed infants, a decision to discontinue breast feeding or the drug should be made, taking into account the importance of the drug to the mother.
Paediatrics
Safety and effectiveness in children have not been established.
INTERACTIONS :
Alcohol
Total body clearance of procainamide is increased by alcohol, but the clinical implications of this are not known.
Amiodarone
Concomitant use may result in increased plasma procainamide and N-acetylprocainamide concentrations and subsequent toxicity. The dosage of intravenous procainamide should be reduced by 20 % to 30 % during concomitant administration. In addition, additive electrophysiologic effects may occur during concomitant use with agents that prolong the QT interval.
Antiarrhythmic agents (other)
If other antiarrhythmic drugs such as lignocaine, phenytoin, propranolol or quinidine are being used, additive or antagonistic effects on the heart may occur with procainamide administration; toxicity may be additive, and dosage reduction may be necessary.
Anticholinergic drugs
Administered concurrently with procainamide may produce additive antivagal effects on A-V nodal conduction, although this is not as well documented for procainamide as for quinidine.
Hypotensive drugs
May cause possible additive hypotensive effect with parenteral doses or high oral doses of procainamide.
Neuromuscular blocking agents : Patients taking procainamide who require neuromuscular blocking agents (eg, succinylcholine) may require less than usual doses of neuromuscular blocking agents.
Trimethoprim
The renal clearance of procainamide and N-acetylprocainamide is reduced by trimethoprim, resulting in increased pharmacodynamic response.
Drug/Laboratory Test Interactions
Suprapharmacologic concentrations of lignocaine and meprobamate may inhibit fluorescence of procainamide and N-acetylprocainamide, and propranolol shows native fluorescence close to the procainamide /N-acetylprocainamide peak wavelengths, so that tests which depend on fluorescence measurement may be affected.
SIDE EFFECTS :
Cardiovascular System :
Hypotension and serious disturbances of cardiorhythm such as ventricular asystole or fibrillation are more common with intravenous administration of procainamide than with intramuscular administration. Because procainamide is a peripheral vasodilator in concentrations higher than the usual therapeutic range, transient high plasma levels which may occur especially during intravenous administration may produce temporary but at times severe lowering of blood pressure.
Multisystem :
A lupus erythematosus-like syndrome of arthralgia, pleural or abdominal pain, and sometimes arthritis, pleural effusion, pericarditis, fever, chills, myalgia, and possibly related haematologic or skin lesions is fairly common after prolonged procainamide administration, perhaps more often in patients who are slow acetylators. While some series have reported less than 1 in 500, others have reported the syndrome in up to 30 percent of patients on long term oral procainamide therapy. If discontinuation of procainamide does not reverse the lupoid symptoms, corticosteroid treatment may be effective.
Haematologic :
Neutropaenia, thrombocytopaenia, or haemolytic anaemia may rarely be encountered. Agranulocytosis has occurred after repeated use of procainamide, and deaths have been reported.
Skin :
Angioneurotic oedema, urticaria, pruritus, flushing, and maculopapular rash have also occurred.
Gastrointestinal System :
Anorexia, nausea, vomiting, abdominal pain, diarrhoea or bitter taste may occur in 3 to 4 percent of patients taking oral procainamide.
Nervous System :
Dizziness or giddiness, weakness, mental depression and psychosis with hallucinations have been reported.
Elevated Liver Enzymes :
Elevations of transaminase with and without elevations of alkaline phosphatase and bilirubin have been reported. Some patients have had clinical symptoms (e.g., malaise, right upper quadrant pain). Deaths from liver failure have been reported.
INFORMATION FOR PATIENTS :
The patient should be encouraged to disclose any past history of drug sensitivity, especially to procaine or other local anesthetic agents, or aspirin, and to report any history of kidney disease, congestive heart failure, myasthenia gravis, liver disease, or lupus erythematosus. The patient should be counseled to report any symptoms of arthralgia, myalgia, fever, chills, skin rash, easy bruising, sore throat or sore mouth, infections, dark urine or icterus, wheezing, muscular weakness, chest or abdominal pain, palpitations, nausea, vomiting, anorexia, diarrhoea, hallucinations, dizziness, or depression.
OVERDOSAGE :
Symptoms of overdose are progressive widening of the QRS complex, prolonged P-R and Q-T intervals, lowering of the R and T waves, as well as increasing A-V block. Increased ventricular extrasystoles, or even ventricular tachycardia or fibrillation, may occur. Transient high plasma levels of procainamide may induce hypotension after intravenous administration, but seldom after oral therapy; this hypotension affects systolic more than diastolic pressures, especially in hypertensive patients. Such high levels may also produce central nervous system depression, tremor, and even respiratory depression. Plasma levels above 10 mcg/ml are increasingly associated with toxic findings, which are seen occasionally in the 10 to 12 mcg/ml range, more often in the 12 to 15 mcg/ml range, and commonly in patients with plasma levels greater than 15 mcg/ml.
TREATMENT OF OVERDOSAGE :
Treatment of overdosage or toxic manifestations includes general supportive measures, close observation, monitoring of vital signs and possibly intravenous pressor agents and mechanical cardiorespiratory support. It can usually be treated, if necessary, by administering vasopressors after adequate fluid volume replacement; IV infusion of 1/6 M sodium lactate injection reportedly reduces the cardiotoxic effects of procainamide. If available, procainamide and N-acetylprocainamide plasma levels may be helpful in assessing the potential degree of toxicity and response to therapy. Both procainamide and N-acetylprocainamide are removed from the circulation by haemodialysis but not by peritoneal dialysis. No specific antidote for procainamide is known.
PHARMACEUTICAL PRECAUTIONS :
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use the Injection if it is darker than slightly yellow or is discoloured in any other way.
STORAGE :
Store below 30°C (86°F), protected from light.
Do not refrigerate.
SHELF LIFE :
24 months from the date of manufacture.
PRESENTATION :
PROCAINAMIDE HYDROCHLORIDE INJECTION USP is supplied as 100 mg Procainamide Hydrochloride USP in 1 ml amber glass ampoule.
Each 1 ampoule is packed in a carton with package insert.
Such 10 cartons are packed in a outer carton.
Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.

 Cardiovascular
Cardiovascular