10 mg/50 ml
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
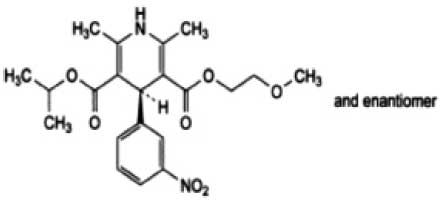
NIMODIPINE INTRAVENOUS INFUSION B.P. is a dihydropyridine calcium channel blocker. Chemically, Nimodipine is 2-Methoxyethyl 1-methylethyl (4RS)-2, 6-dimethyl-4-(3-nitrophenyl)-1,4-dihydropyridine-3, 5-dicarboxlate. Its molecular formula is C21H26N2O7 and molecular weight is 418.4.
STRUCTURAL FORMULA :
Its structural formula is :
NIMODIPINE INTRAVENOUS INFUSION B.P. is a sterile, clear, yellow, solution for infusion filled in a vial of suitable size.
COMPOSITION :
Each 50 ml contains :
Nimodipine B.P. 10 mg
Ethanol B.P. 20 % w/v
Water for Injections B.P. q.s.
Contains no preservatives
Administration :
NIMODIPINE INTRAVENOUS INFUSION B.P. is administered as a continuous I.V. infusion via a central catheter using an infusion pump. It should be connected to a three-way stopcock using the infusion line provided. The three-way stopcock should be used to connect the Nimodipine polyethylene tube with the co-infusion line and the central catheter. (The stopcock must allow for concomitant flow of the NIMODIPINE INTRAVENOUS INFUSION B.P. and a co-infusion solution.) NIMODIPINE INTRAVENOUS INFUSION B.P. must be administered with a co-infusion running at a rate of 40 ml/hr of either sodium chloride 0.9 %, glucose 5 %, Ringer’s lactate solution, lactated Ringer’s solution with magnesium, dextran 40, (poly[O-2-hydroxyethyl]) starch 6 %, human albumin 5 %, blood or mannitol 10 % in a ratio of about 1:4 (Nimodipine : co-infusion), which is connected to the second port of the three-way stopcock prior to its connection with the central line catheter.
NIMODIPINE INTRAVENOUS INFUSION B.P. must not be added to an infusion bag or bottle and must not be mixed with other drugs. NIMODIPINE INTRAVENOUS INFUSION B.P. may be used during anaesthesia, angiography or surgical procedures. Polyvinyl Chloride (PVC) equipment should not be used for administration of NIMODIPINE INTRAVENOUS INFUSION B.P.
Dosage :
Recommended dose - Aneurysmal Subarachnoid Haemorrhage :
For the first two hours of treatment 1 mg of Nimodipine, i.e. 5 ml NIMODIPINE INTRAVENOUS INFUSION B.P., (about 15 μg/kg bw/h), should be infused each hour via a central catheter. If it is well tolerated, the dose should be increased after two hours to 2 mg Nimodipine, i.e. 10 ml NIMODIPINE INTRAVENOUS INFUSION B.P. per hour (about 30 μg/kg bw/h), providing no severe decrease in blood pressure is observed. Patients of body weight less than 70 kg or with unstable blood pressure should be started on a dose of 0.5 mg nimodipine per hour (2.5 ml of Nimodipine solution), or less if necessary.
Duration of treatment :
Aneurysmal subarachnoid haemorrhage Intravenous treatment should begin as early as possible after neurological deficit occurs due to arterial spasm, post subarachnoid haemorrhage. This should continue for at least five days up to a maximum of 14 days. In the event of surgical intervention during treatment, administration of Nimodipine should be continued (dose as above) for at least five days. NIMODIPINE INTRAVENOUS INFUSION B.P. may be used with or without pre-treatment with Nimodipine tablets. In the event of NIMODIPINE INTRAVENOUS INFUSION B.P. being administered sequentially the total duration of treatment should not exceed 21 days. NIMODIPINE INTRAVENOUS INFUSION B.P. should not be administered for longer than 14 days. NIMODIPINE INTRAVENOUS INFUSION B.P. should not be used concomitantly.
Traumatic subarachnoid haemorrhage
Not recommended as a positive benefit to risk ratio has not been established.
CONTRAINDICATIONS :
Known hypersensitivity to NIMODIPINE INTRAVENOUS INFUSION B.P. or any of the excipients. Nimodipine should not be administered to patients during or within one month of a myocardial infarction or an episode of unstable angina.
WARNINGS :
Although treatment with Nimodipine has not been shown to be associated with increases in intracranial pressure, close monitoring is recommended in these cases or when the water content of the brain tissue is elevated (generalized cerebral oedema). Caution is required in patients with hypotension (systolic blood pressure lower than 100 mm Hg).
PRECAUTIONS :
General :
Blood Pressure :
Nimodipine has the haemodynamic effects expected of a calcium channel blocker, although they are generally not marked. However, intravenous administration of the contents of NIMODIPINE INTRAVENOUS INFUSION B.P. has resulted in serious adverse consequences including death, cardiac arrest, cardiovascular collapse, hypotension, and bradycardia. In patients with subarachnoid haemorrhage given NIMODIPINE INTRAVENOUS INFUSION B.P. in clinical studies, about 5 % were reported to have had lowering of the blood pressure and about 1 % left the study because of this (not all could be attributed to nimodipine). Nevertheless, blood pressure should be carefully monitored during treatment with NIMODIPINE INTRAVENOUS INFUSION B.P. based on its known pharmacology and the known effects of calcium channel blockers.
Hepatic Disease :
The metabolism of NIMODIPINE INTRAVENOUS INFUSION B.P. is decreased in patients with impaired hepatic function. Such patients should have their blood pressure and pulse rate monitored closely and should be given a lower dose. Intestinal pseudo-obstruction and ileus have been reported rarely in patients treated with nimodipine. A causal relationship has not been established. The condition has responded to conservative management.
Laboratory Test Interactions :
None known.
Pregnancy : Pregnancy Category C
NIMODIPINE INTRAVENOUS INFUSION B.P. has been shown to have a teratogenic effect in Himalayan rabbits. Incidences of malformations and stunted foetuses were increased at oral doses of 1 and 10 mg/kg/day administered (by gavage) from day 6 through day 18 of pregnancy but not at 3.0 mg/kg/day in one of two identical rabbit studies. In the second study an increased incidence of stunted foetuses was seen at 1.0 mg/kg/day but not at higher doses. Nimodipine was embryotoxic, causing resorption and stunted growth of foetuses, in Long Evans rats at 100 mg/kg/day administered by gavage from day 6 through day 15 of pregnancy. In two other rat studies, doses of 30 mg/kg/day nimodipine administered by gavage from day 16 of gestation and continued until sacrifice (day 20 of pregnancy or day 21 post partum) were associated with higher incidences of skeletal variation, stunted foetuses and stillbirths but no malformations. There are no adequate and well controlled studies in pregnant women. If nimodipine is to be administered during pregnancy, the benefits and the potential risks must therefore be carefully weighed according to the severity of the clinical picture.
Lactation :
Nimodipine and/or its metabolites have been shown to appear in rat milk at concentrations much higher than in maternal plasma. Nimodipine and its metabolites have been shown to appear in human milk at concentrations of the same order of magnitude as corresponding maternal plasma concentrations. Nursing mothers are advised not to breastfeed their babies when taking the drug.
Paediatric Use :
Safety and effectiveness in children have not been established.
INTERACTIONS :
Drugs that affect nimodipine Concurrent twice daily administration of 30 mg nimodipine and daily administration of 20 mg of the antidepressant fluoxetine to elderly patients resulted in an increase in nimodipine plasma levels, a reduction in fluoxetine levels and a trend towards increased norfluoxetine levels. The daily dose used in patients with subarachnoid haemorrhage is four times the daily dose used in this trial, and as a steady state norfluoxetine level was not achieved, the clinical significance of this interaction in the treatment of aneurysmal subarachnoid haemorrhage (aSAH) is uncertain. Concurrent three times daily administration of 30 mg nimodipine and three times daily administration of 10 mg of the antidepressant nortriptyline to elderly patients resulted in a slight decrease in nimodipine plasma levels with no effect on nortriptyline plasma levels. The daily dose used in patients with subarachnoid haemorrhage is four times the daily dose used in this trial, thus the clinical significance of this interaction in the treatment of aneurysmal subarachnoid haemorrhage (aSAH) is uncertain. Nimodipine is metabolised via the cytochrome P450 3A4 system, located both in the intestinal mucosa and in the liver. Although no formal interaction studies have been performed to investigate the potential interaction between nimodipine and inhibitors of cytochrome P450 3A4, the potential for drug interaction and increased nimodipine plasma concentrations cannot be excluded.
Effects of nimodipine on other drugs
Blood pressure lowering drugs Nimodipine may increase the blood pressure lowering effect of concomitant antihypertensives, such as diuretics, beta-blockers, ACE inhibitors, A1-antagonists, other calcium antagonists, alpha-adrenergic blocking agents, PDE5 inhibitors and alpha-methyldopa. If a combination of this type proves unavoidable, particularly careful monitoring of the patient is necessary. Simultaneous intravenous administration of beta-blockers may lead to mutual potentiation of negative inotropic action going as far as
decompensated heart failure Renal function can deteriorate if potentially nephrotoxic drugs (e.g. aminoglycosides, cephalosporins, furosemide) are given simultaneously and also in patients whose renal function is already impaired. Renal function must be monitored carefully in such cases and if deterioration is found discontinuation of the treatment should be considered. Animal studies have shown that when nimodipine and zidovudine are administered concomitantly, the AUC for zidovudine was increased, and the volume of distribution and clearance rate decreased. The clinical relevance of this interaction is unknown, but since the side-effect profile of zidovudine is known to be dose related, this interaction should be considered in patients receiving nimodipine and zidovudine concomitantly.
Other forms of interaction
Since NIMODIPINE INTRAVENOUS INFUSION B.P. contains 20 vol % ethanol (alcohol), patients should be monitored for any possible interactions with alcohol-incompatible drugs. The simultaneous administration of cimetidine or sodium valproate may lead to an increase in the plasma nimodipine concentration.The intake of grapefruit juice is not recommended in combination with nimodipine as it can result in increased plasma nimodipine concentrations due to the inhibition of the oxidative metabolism of dihydropyridines.
SIDE EFFECTS :
The following events have been mainly reported in clinical trials. The following definitions of frequencies are used :
Very common (≥ 1/10); Common (≥ 1/100 to < 1/10); Uncommon (≥ 1/1,000 to ≤1/100); Rare (≥ 1/10,000 to ≤ 1/1,000); Very rare (≤ 1/10,000)
Blood and Lymphatic System Disorders :
Thrombocytopenia is uncommon.
Immune System Disorders :
• Acute hypersensitivity reactions include uncommonly occurring mild to moderate allergic reactions.
• Associated clinical symptoms related to skin (uncommon rash).
Nervous System Disorders :
Unspecific cerebrovascular symptoms include uncommonly occurring headache.
Cardiac Disorders :
Unspecific cardiac arrhythmias: tachycardia is uncommon and bradycardia is rare.
Vascular Disorders :
Unspecific cardiovascular symptoms such as hypotension and vasodilatation (sweating, flushing and feeling of warmth) are uncommon.
Gastrointestinal Disorders :
• Unspecific gastrointestinal and abdominal symptoms include uncommonly occurring nausea.
• Rarely ileus has been reported.
Haepatobiliary Disorders :
Liver reactions include a rarely occurring transient increase in liver enzymes (including an increase in transaminases, alkaline phosphatase and γ-GT).
General Disorders and Administration Site Conditions :
Infusion and injection site reactions are rare (including infusion site (thrombo-) phlebitis).
OVERDOSAGE :
Symptoms of acute overdosage to be anticipated are marked lowering of the blood pressure, tachycardia, bradycardia and (after oral administration) gastro-intestinal complaints and nausea.
TREATMENT OF OVERDOSAGE :
In the event of acute overdosage, treatment with NIMODIPINE INTRAVENOUS INFUSION B.P. must be discontinued immediately. Emergency measures should be governed by the symptoms. Gastric lavage with addition of charcoal should be considered as an emergency therapeutic measure. If there is a marked fall in blood pressure, dopamine or noradrenaline can be administered intravenously.
PHARMACEUTICAL PRECAUTIONS :
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
STORAGE :
Store below 30°C (86°F), protected from light.
Do not refrigerate.
SHELF LIFE :
24 months from the date of manufacture.
PRESENTATION :
NIMODIPINE INTRAVENOUS INFUSION B.P. contains Nimodipine 10 mg in 50 ml solution.
Single vial pack.
Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.

 Cardiovascular
Cardiovascular