10 mg
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
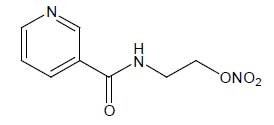
Nicorandil Tablets (Nicorandil) is a Nicotinamide derivative; exhibits dual mechanism of action as both nitrovasodilator and potassium channel activator. Chemically, Nicorandil is N-[2-(Nitroxy) ethyl]-3-pyridinecarboxamide.The molecular formula is C8H9N3O4 and molecular weight is 211.2.
STRUCTURAL FORMULA :
Its structural formula is :
NICORANDIL TABLETS is white coloured, circular, biconvex tablets having score line on one side and “SGP” embossed on other side of each tablet.
COMPOSITION :
Each uncoated tablet contains :
Nicorandil 10 mg
Excipients q.s.
ACTIONS :
Nicorandil provides a dual mode of action leading to relaxation of vascular smooth muscle. A potassium channel opening action provides arterial vasodilation, thus reducing afterload, while the nitrate component promotes venous relaxation and a reduction in preload. Nicorandil has a direct effect on coronary arteries without leading to a steal phenomenon. The overall action improves blood flow to post-stenotic regions and the oxygen balance in the myocardium.
PHARMACOKINETICS :
Nicorandil is well absorbed from the gastrointestinal tract and maximum plasma concentrations are achieved 30 to 60 minutes after administration by mouth Metabolism is mainly by denitration and about 20 % of a dose is excreted in the urine mainly as metabolites. The elimination half-life is about 1 hour. Nicorandil is only slightly bound to plasma proteins.
Administration : For oral use
Dosage :
The usual initial dose by mouth is 10 mg twice daily (or 5 mg twice daily in patients susceptible to headache), increased as necessary to a maximum of 30 mg twice daily; the usual therapeutic dose is in the range of 10 to 20 mg twice daily.
Elderly :
For elderly patients use of the lowest effective dose is recommended.
Children :
A paediatric dosage has not been established and use of nicorandil is not recommended.
CONTRAINDICATIONS :
NICORANDIL TABLETS is contra-indicated in
• known or idiosyncratic hypersensitivity to nicorandil, nicotinamide and nicotinic acid
• cardiogenic shock
• acute myocardial infarction with acute left ventricular failure and low filling pressures
• hypotension
Due to the risk of severe hypotension, the concomitant use of nicorandil and phosphodiesterase 5 inhibitors (e.g. sildenafil, tadalafil, vardenafil) is contraindicated. NICORANDIL TABLETS contain lactose which is contra-indicated in patients with galactosaemia,the glucose-galactose malabsorption syndrome, or lactase deficiency.
WARNINGS AND PRECAUTIONS :
Gastrointestinal ulcerations, skin and mucosal ulceration have been reported with nicorandil. These are refractory to treatment and most only respond to withdrawal of nicorandil treatment. If ulcerations develop, nicorandil should be discontinued. Gastrointestinal perforations in context of concomitant use of nicorandil and corticosteroids have been reported. Caution is advised when concomitant use is considered. Nicorandil must be used with caution in patients who may have blood volume depletion or in those who present, low systolic blood pressure (e.g. below 100 mm Hg), acute pulmonary oedema or acute myocardial infarction with acute left ventricular failure and low filling pressures. Nicorandil may lower the blood pressure of hypertensive patients and therefore should be used with care when prescribed with antihypertensive drugs. Caution is advised if nicorandil is used in combination with other medicinal products with blood pressure lowering effect. Caution is advised for the use of nicorandil in patients with glaucoma. The hypotensive effect of other vasodilators, tricyclic antidepressants or alcohol can be increased by administration in combination with nicorandil. NICORANDIL TABLETS should be used cautiously in diabetic patients.
Pregnancy : Category B3.
Nicorandil has not been studied in pregnant women. Although animal studies have shown that nicorandil is not teratogenic, it has been shown to increase pre-implantation loss at oral doses of 40 mg/kg/day in rats and to increase foetal mortality at doses of 100 mg/kg/day. The significance of these findings is unknown. Nicorandil should not be used during pregnancy unless it is considered essential by the physician.
Nursing mothers :
It is not known whether nicorandil is excreted in milk. Animal studies have shown that nicorandil increases perinatal mortality at 50 mg/kg/day. The significance of this finding to human use is unclear. Thus, nicorandil is not recommended for use during breast feeding.
INTERACTIONS AND INCOMPATIBILITIES :
Nicorandil has the interaction with following drugs :
Alcohol
Hypotensive effect of nicorandil possibly enhanced by alcohol.
Antidepressants, Tricyclic
Hypotensive effect of nicorandil possibly enhanced by tricyclics
Hydralazine
Possible enhanced hypotensive effect when nicorandil given with hydralazine.
MAOIs
Enhanced hypotensive effect when nicorandil given with MAOIs
Minoxidil
Possible enhanced hypotensive effect when nicorandil given with minoxidil.
Sildenafil
Hypotensive effect of nicorandil significantly enhanced by sildenafil (avoid concomitant use).
Sodium Nitroprusside
Possible enhanced hypotensive effect when nicorandil given with sodium nitroprusside.
Tadalafil
Hypotensive effect of nicorandil significantly enhanced by tadalafil (avoid concomitant use).
Vardenafil
Possible increased Hypotensive effect when nicorandil given with vardenafil (avoid concomitant use)
OVERDOSAGE :
In case of acute overdose, the likely symptomatology may be peripheral vasodilation with a fall in blood pressure and reflex tachycardia. There is no experience of massive overdosage in humans.
TREATMENT OF OVERDOSAGE :
Monitoring cardiac function and general supportive measures are recommended. If not successful, increase in circulating plasma volume by substitution of fluid is recommended. In life-threatening situations, administration of vasopressors must be considered.
STORAGE :
Store below 25°C (77°F), protected from moisture and light.
Do not refrigerate.
SHELF LIFE :
24 months from the date of manufacture.
PRESENTATION :
NICORANDIL TABLETS contains Nicorandil 10 mg.
3 blisters of 10 tablets per box.
Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.

 Cardiovascular
Cardiovascular