
0.5 mg + 2.5 mg/ml
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
GLYCOPYRROLATE AND NEOSTIGMINE METHYLSULFATE INJECTION is an anticholinergic (antimuscarinic) agent. Chemically, Glycopyrrolate is Pyrrolidinium,3-[(SR)-(cyclopentylhydroxyphenylacetyl)oxy]-1,1-dimethyl-, [RS-] bromide. The molecular formula is C19H28BrNO3 and molecular weight is 398.33. Chemically, Neostigmine Methylsulfate is Benzenaminium, 3-[[(dimethylamino)carbonyl]oxy]-N,N,N-trimethyl-,methyl sulfate. The molecular formula is C13H22N2O6S and molecular weight is 334.39.
STRUCTURAL FORMULA :
Its structural formula is :
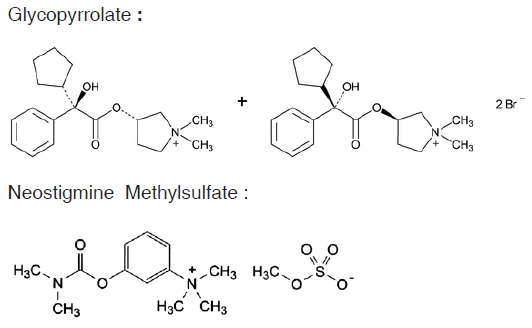
GLYCOPYRROLATE AND NEOSTIGMINE METHYLSULFATE INJECTION is a sterile, clear, colourless solution filled in amber ampoule of suitable size.
COMPOSITION :
Each ml contains :
Glycopyrrolate USP 0.5 mg
Neostigmine Methylsulfate USP 2.5 mg
Water for Injection USP q.s.
ACTIONS :
Glycopyrrolate is a quaternary ammonium antimuscarinic agent and like other anticholinergic agents, it inhibits the action of acetylcholine on structures innervated by postganglionic cholinergic nerves and on smooth muscles that respond to acetylcholine but lack cholinergic innervation. These peripheral cholinergic receptors are present in the autonomic effector cells of smooth muscle, cardiac muscle, the sinoatrial node, the atrioventricular node, exocrine glands and to a limited degree in the autonomic ganglia. Thus it diminishes the volume and free acidity of gastric secretions and controls excessive pharyngeal, tracheal and bronchial secretions. Glycopyrrolate antagonizes muscarinic symptoms (e.g. bronchorrhea, bronchospasm, bradycardia and intestinal hypermotility) induced by cholinergic drugs such as the anticholinesterases. The highly polar quaternary ammonium group of glycopyrrolate limits its passage across lipid membranes, such as the blood-brain barrier, in contrast to atropine sulphate and scopolamine hydrobromide, which are non-polar tertiary amines which penetrate lipid barriers easily. Neostigmine inhibits the hydrolysis of acetylcholine by competing with acetylcholine for attachment to acetylcholinesterase at sites of cholinergic transmission. It enhances cholinergic action by facilitating the transmission of impulses across neuromuscular junctions. It also has a direct cholinomimetic effect on skeletal muscle and possibly on automatic ganglion cells and neurons of the central nervous system.
PHARMACOKINETICS :
Glycopyrrolate and Neostigmine Methylsulfate are routinely administered simultaneously to reverse residual non-depolarising (competitive) neuromuscular block. Numerous clinical studies, which demonstrate this to be a safe and effective combination, have been published. Over 90 % of the Glycopyrrolate disappears from serum within 5 minutes following intravenous administration. The drug is rapidly excreted into bile with highest concentrations being found 30 to 60 minutes after dosing with some product being detected up to 48 hours after administration. Glycopyrrolate is also rapidly excreted into urine with the highest concentrations being found within 3 hours of administration. Over 85 % of product is excreted within 48 hours. It has subsequently been confirmed in a single dose pharmacokinetic study using radio immunological assay procedures that Glycopyrrolate was rapidly distributed and/or excreted after intravenous administration. The terminal elimination phase was relatively slow with quantifiable plasma levels remaining up to 8 hours after administration. The elimination half-life was 1.7 hours. Neostigmine Methylsulfate is extensively hydrolyzed in the blood. In one study, following intravenous administration, the plasma concentration declined to about 8 % of its initial value after 5 minutes with a distribution half-life of less than one minute. Elimination half-life ranged from about 15 - 30 minutes. Trace amounts of Neostigmine Methylsulfate could be detected in the plasma after one hour. In a study in non-myasthenic patients, the plasma half-life was 0.89 hours.
INDICATIONS :
GLYCOPYRROLATE AND NEOSTIGMINE METHYLSULFATE INJECTION is indicated for reversal of residual non-depolarising (competitive) neuromuscular block.
Administration :
GLYCOPYRROLATE AND NEOSTIGMINE METHYLSULFATE INJECTION is for intravenous administration.
INSTRUCTIONS FOR USE OF AMPOULE :
The ampoule used in this product is equipped with O.P.C. (One Point Cut) opening system. No ampoule file is needed to open the ampoule. The neck of the ampoule is prescored at the point of constriction. A coloured dot on the ampoule head helps to orientate the ampoule. Take the ampoule and face the coloured dot. Let the solution at the head of the ampoule to flow down by shaking or a gentle stroke. The ampoule opens easily by placing the thumb on the coloured dot and gently pressing downwards as shown.

Dosage :
Adults and older patients :
1 - 2 ml intravenously over a period of 10 - 30 seconds [equivalent to neostigmine methylsulfate 2500 micrograms (2.5 mg) with glycopyrrolate 500 micrograms (0.5 mg) to neostigmine methylsulfate 5000 micrograms (5 mg) with glycopyrrolate 1000 micrograms (1 mg)].
Alternatively 0.02 ml/kg intravenously over a period of 10 - 30 seconds may be used [equivalent to neostigmine methylsulfate 50 micrograms/kg (0.05 mg/kg) with glycopyrrolate 10 micrograms/kg (0.01mg/kg)].
Children :
0.02 ml/kg intravenously over a period of 10 - 30 seconds [equivalent to neostigmine methylsulfate 50 micrograms/kg (0.05 mg/kg) with glycopyrrolate 10 micrograms/kg (0.01 mg/kg)]. These doses may be repeated if adequate reversal of neuromuscular blockade is not achieved. Total doses in excess of 2 ml are not recommended as this dose of neostigmine may produce depolarising neuromuscular block.
CONTRAINDICATIONS :
GLYCOPYRROLATE AND NEOSTIGMINE METHYLSULFATE INJECTION should not be given to patients with known hypersensitivity to either of the two active ingredients or any of the excipients. GLYCOPYRROLATE AND NEOSTIGMINE METHYLSULFATE INJECTION should not be given in conjunction with suxamethonium as neostigmine potentiates the depolarising myoneural blocking effects of this agent.
Administer with caution to patients with bronchospasm, or severe bradycardia. Administration of anticholinesterase agents to patients with intestinal anastomosis may produce rupture of the anastomosis or leakage of intestinal contents. Although GLYCOPYRROLATE AND NEOSTIGMINE METHYLSULFATE INJECTION has been shown to have less impact on the cardiovascular system than atropine with neostigmine methylsulfate, use with caution in patients with coronary artery disease, congestive heart failure, cardiac dysrhythmias, hypertension, thyrotoxicosis and cardiac insufficiency. Use with caution in patients with epilepsy or Parkinsonism. As glycopyrrolate inhibits sweating, patients with increased temperature (especially children) should be observed closely.
In common with other antimuscarinic drugs caution is advised in patients with prostatic hypertrophy, paralytic ileus, pyloric stenosis and closed angle glaucoma. Anticholinergic drugs can cause ventricular arrhythmias when administered during inhalation anaesthesia especially in association with the halogenated hydrocarbons. Quaternary ammonium compounds in large dose have been shown to block the nicotinic muscle end plate receptors. This must be evaluated prior to its administration in patients with myasthenia gravis. Unlike atropine, glycopyrrolate is a quaternary ammonium compound and does not cross the blood-brain barrier. It is therefore less likely to cause postoperative confusion which is a particular concern in the elderly patients. Compared to atropine, glycopyrrolate has reduced cardiovascular and ocular effects. Neostigmine methylsulfate : Glycopyrronium or alternatively atropine, given before or with neostigmine, prevents bradycardia, excessive salivation, and other muscarinic effects of neostigmine.
Pregnancy : Category C
Teratogenic Effects :
There are no adequate or well-controlled studies of neostigmine in either laboratory animals or in pregnant women. It is not known whether neostigmine can cause foetal harm when administered to pregnant women or can affect reproductive capacity. Neostigmine should be given to pregnant women only if clearly needed.
Nonteratogenic effects :
Anticholinesterase drugs may cause uterine irritability and induce premature labor when given intravenously to pregnant women near term.
Nursing mothers :
It is not known whether neostigmine is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions from neostigmine in nursing infants, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Paediatric use :
Safety and effectiveness in children have not been established.
INTERACTIONS :
The effects of atropine and other antimuscarinics may be enhanced by use with other drugs having antimuscarinic properties, such as amantadine, some antihistamines, phenothiazine antipsychotics, and tricyclic antidepressants. Inhibition of drug-metabolising enzymes by MAOIs may possibly enhance the effects of antimuscarinics. The reduction in gastric motility caused by antimuscarinics may affect the absorption of other drugs. Antimuscarinics may also antagonise the gastrointestinal effects of cisapride, domperidone, and metoclopramide. Antimuscarinics and parasympathomimetics may counteract each others effects. Concomitant administration of glycopyrrolate and potassium chloride in a wax matrix may increase the severity of potassium chloride-induced gastrointestinal lesions as a result of a slower gastrointestinal transit time. Drugs with neuromuscular blocking activity, such as the aminoglycosides, clindamycin, colistin, cyclopropane, and the halogenated inhalational anaesthetics, may antagonise the effects of neostigmine. A number of drugs, including quinine, chloroquine, hydroxychloroquine, quinidine, procainamide, propafenone, lithium, and the beta blockers, that have the potential to aggravate myasthenia gravis can reduce the effectiveness of treatment with parasympathomimetics. Prolonged bradycardia has also occurred in patients receiving beta blockers when given neostigmine. Anticholinesterases, such as neostigmine, can inhibit the metabolism of suxamethonium and enhance and prolong its action. Ophthalmic use of anticholinesterases, such as ecothiopate, should be undertaken with care in patients receiving neostigmine systemically for myasthenia gravis, because of possible additive toxicity. Antimuscarinics such as atropine antagonise the muscarinic effects of neostigmine.
Beta blockers :
There have been several reports of bradycardia and hypotension when neostigmine or physostigmine were given to patients receiving beta blockers but no significant changes in heart rate were noted in a study of pyridostigmine given to 8 patients taking beta blockers. Beta blockers have the potential to aggravate the symptoms of myasthenia gravis and may therefore reduce the effectiveness of parasympathomimetic treatment.
Calcium-channel blockers :
Use of calcium-channel blockers such as verapamil with neuromuscular blockers may produce an enhanced muscle block which is resistant to reversal with neostigmine but which can be reversed by edrophonium.
Corticosteroids :
Although use of glucocorticoids alone may improve strength in myasthenic patients, use of methylprednisolone in patients receiving neostigmine or pyridostigmine has exacerbated symptoms and produced profound weakness needing assisted ventilation. Since the adverse effects of combined therapy usually occur before any expected benefits it has been suggested that the glucocorticoid should be given on alternate days in small doses which are increased gradually until the optimal effect is achieved.
INCOMPATIBILITIES :
Do not mix GLYCOPYRROLATE AND NEOSTIGMINE METHYLSULFATE INJECTION with any other product or preparation.
SIDE EFFECTS :
The glycopyrrolate component of GLYCOPYRROLATE AND NEOSTIGMINE METHYLSULFATE INJECTION can give rise to dry mouth, constipation, transient bradycardia (followed by tachycardia, palpitation and arrhythmias), reduced bronchial secretions, urinary urgency and retention, dilatation of the pupils, photophobia, flushing and dryness of the skin. Side effects that occur occasionally include confusion (particularly in the elderly), nausea, vomiting, and giddiness~ very rarely, angle-closure glaucoma may occur. The neostigmine component of GLYCOPYRROLATE AND NEOSTIGMINE METHYLSULFATE INJECTION can give rise to bradycardia, increased oropharyngeal secretions, cardiac dysrhythmias and increased gastrointestinal activity. If severe neostigmine-induced muscarinic side effects occur (bradycardia, increased oropharyngeal secretions, decreased cardiac conduction rate, increased sweating, bronchospasm or increased gastrointestinal activity etc), these may be treated by the intravenous administration of Glycopyrrolate Injection 200 - 600 micrograms (0.2 - 0.6 mg) or atropine 400 - 1200 micrograms (0.4 - 1.2 mg).
EFFECTS ON ABILITY TO DRIVE AND USE MACHINES :
Not relevant as the product is for intra-operative use.
OVERDOSAGE AND TREATMENT OF OVERDOSAGE :
The treatment of overdosage depends upon whether signs of anticholinesterase or anticholinergic overdosage are predominant presenting features. Signs of neostigmine overdosage include nausea, vomiting, diarrhoea, excessive salivation and sweating, increased oropharyngeal secretions, miosis, bradycardia or tachycardia, cardiospasm, bronchospasm, incoordination, muscle cramps, fasciculation and paralysis.This may be treated by the administration of Glycopyrrolate Injection 200 - 600 micrograms (0.2 - 0.6 mg) or atropine 400 - 1200 micrograms (0.4 - 1.2 mg). In severe cases, respiratory depression may occur and artificial ventilation may be necessary in such patients. Signs of glycopyrrolate overdosage (tachycardia, ventricular irritability etc) may be treated by the administration of neostigmine methylsulfate 1000 micrograms (1.0 mg) for each 1000 micrograms (1.0 mg) of glycopyrrolate known to have been administered. As glycopyrrolate is a quaternary ammonium agent, symptoms of overdosage are peripheral rather than central in nature; centrally acting anticholinesterase drugs such as physostigmine are therefore unnecessary to treat glycopyrrolate overdosage.
PHARMACEUTICAL PRECAUTIONS :
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
STORAGE :
Store below 30°C (86°F), protected from light.
Do not refrigerate.
SHELF LIFE :
24 months from the date of manufacture.
PRESENTATION :
GLYCOPYRROLATE AND NEOSTIGMINE METHYLSULFATE INJECTION is supplied as 0.5 mg of Glycopyrrolate USP and 2.5 mg of Neostigmine Methylsulfate USP in 1 ml aqueous solution.
Such 1 Ampoule is packed in a Box.
Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.

 Cardiovascular
Cardiovascular





