
100 mg, 400 mg
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
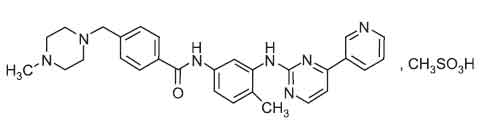
IMATINIB CAPSULES I.P. (Imatinib Mesylate) is a tyrosine kinase inhibitor that inhibits the BCR-ABL tyrosine kinase. Chemically, Imatinib Mesylate is 4-[(4-methyl - 1 - piperazinyl)methyl] –N [4-methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl] amino]phenyl] benzamide methanesulphonate. The molecular formula is C29H31N7O,CH3SO3H and molecular weight is 589.7.
STRUCTURAL FORMULA :
Its structural formula is :
IMATINIB CAPSULES I.P. are yellowish crystalline powder filled in hard gelatin capsule of suitable size.
COMPOSITION :
Each hard gelatin capsule contains :
Imatinib Mesylate I.P.
equivalent to Imatinib 100 mg
Excipients q.s.
Approved colours used in empty capsule shell.
Each hard gelatin capsule contains :
Imatinib Mesylate I.P.
equivalent to Imatinib 400 mg
Excipients q.s.
Approved colours used in empty capsule shell.
ACTIONS :
Imatinib is a small molecule protein-tyrosine kinase inhibitor that potently inhibits the activity of the Bcr-Abl tyrosine kinase (TK), as well as several receptor TKs : Kit, the receptor for stem cell factor (SCF) coded for by the c-Kit proto-oncogene, the discoidin domain receptors (DDR1) and (DDR2), the colony stimulating factor receptor (CSF-1R) and the platelet-derived growth factor receptors alpha and beta (PDGFR-alpha and PDGFR-beta). Imatinib can also inhibit cellular events mediated by activation of these receptor kinases.
PHARMACODYNAMICS :
Imatinib is a protein-tyrosine kinase inhibitor, which potently inhibits the breakpoint cluster region - Abelson (Bcr-Abl) tyrosine kinase at the in vitro, cellular, in vivo levels. The compound selectively inhibits proliferation and induces apoptosis in Bcr-Abl positive cell lines as well as fresh leukaemic cells from Philadelphia chromosome positive CML and acute lymphoblastic leukaemia (ALL) patients. In colony transformation assays using ex vivo peripheral blood and bone marrow samples, imatinib shows selective inhibition of Bcr-Abl positive colonies from CML patients. In vivo the compound shows anti-tumour activity as a single agent in animal models using Bcr-Abl positive tumour cells. Imatinib is also an inhibitor of the receptor tyrosine kinases for platelet-derived growth factor (PDGF) and stem cell factor (SCF), c-Kit, and inhibits PDGF- and SCF-mediated cellular events. Constitutive activation of the PDGFR or the Abl protein tyrosine kinases as a consequence of fusion to diverse partner proteins or constitutive production of PDGF have been implicated in the pathogenesis of MDS/MPD, HES/CEL and DFSP. In addition, constitutive activation of c-Kit or the PDGFR has been implicated in the pathogenesis of SM. Imatinib inhibits signalling and proliferation of cells driven by dysregulated PDGFR, Kit and Abl kinase activity.
PHARMACOKINETICS :
Imatinib mesylate is well absorbed after oral doses with peak blood concentrations occurring after 2 to 4 hours. The mean bioavailability is about 98 %. Imatinib is reported to be about 95 % bound to plasma proteins. Plasma elimination half-lives of imatinib and its major active metabolite, the N-demethylated piperazine derivative, are about 18 and 40 hours respectively. The major enzyme responsible for the metabolism of imatinib is cytochrome P450 isoenzyme CYP3A4; isoenzymes CYP1A2, CYP2D6, CYP2C9, and CYP2C19 also play a minor role. About 81 % of a dose is eliminated within 7 days in the faeces (68 %) and urine (13 %). It is excreted mostly as metabolites, with only 25 % as unchanged drug.
INDICATIONS :
IMATINIB CAPSULES I.P. is indicated for the treatment of patients with chronic myeloid leukaemia (CML) in blast crisis, accelerated phase, or in chronic phase after failure of interferon-alpha therapy. The effectiveness of IMATINIB CAPSULES I.P. is based on overall haematologic and cytogenetic response rates. There are no controlled trials demonstrating a clinical benefit, such as improvement in disease-related symptoms or increased survival.
Administration :
IMATINIB CAPSULES I.P. are for oral administration.
Dosage :
Therapy should be initiated by a physician experienced in the treatment of patients with chronic myeloid leukaemia. The recommended dosage of IMATINIB CAPSULES I.P. are 400 mg/day for patients in chronic phase CML and 600 mg/day for patients in accelerated phase or blast crisis. The prescribed dose should be administered orally, once daily with a meal and a large glass of water. Treatment should be continued as long as the patient continues to benefit. Dose increase from 400 mg to 600 mg in patients with chronic phase disease, or from 600 mg to 800 mg (given as 400 mg twice daily) in patients in accelerated phase or blast crisis may be considered in the absence of severe adverse drug reaction and severe non-leukaemia related neutropenia or thrombocytopaenia in the following
circumstances: disease progression (at any time); failure to achieve a satisfactory haematologic response after at least 3 months of treatment; loss of a previously achieved haematologic response. Dose adjustment for hepatotoxicity and other non-haematologic adverse reactions If a severe non- haematologic adverse reaction develops (such as severe hepatotoxicity or severe fluid retention), IMATINIB CAPSULES I.P. should be withheld until the event has resolved. Thereafter, treatment can be resumed as appropriate depending on the initial severity of the event.
If elevations in bilirubin > 3 x institutional upper limit of normal (IULN) or in liver transaminases > 5 x IULN occur, IMATINIB CAPSULES I.P. should be withheld until bilirubin levels have returned to < 1.5 x IULN and transaminase levels to < 2.5 x IULN. Treatment with
IMATINIB CAPSULES I.P. may then be continued at a reduced daily dose (i.e. 400 → 300 mg or 600 → 400 mg).
Paediatric :
The safety and efficacy of IMATINIB CAPSULES I.P. in patients under the age of 18 years have not been established.
CONTRAINDICATIONS :
Use in patients with a hypersensitivity to the active substance or to any of the excipients is contraindicated.
When IMATINIB CAPSULES I.P. is co-administered with other medications, there is a potential for drug interactions. Caution should be used when taking IMATINIB CAPSULES I.P. with rifampicin or other strong CYP3A4 inducers, ketoconazole or other strong CYP3A4 inhibitors, CYP3A4 substrates with a narrow therapeutic window (e.g. cyclosporin or pimozide) or CYP2C9 substrates with a narrow therapeutic window (e.g. warfarin and other coumarin derivatives).
Hypothyroidism :
Clinical cases of hypothyroidism have been reported in thyroidectomy patients undergoing levothyroxine replacement during treatment with Imatinib. TSH levels should be closely monitored in such patients.
Hepatotoxicity :
In patients with hepatic dysfunction (mild, moderate or severe), peripheral blood counts and liver enzymes should be carefully monitored. When Imatinib is combined with high dose chemotherapy regimens, transient liver toxicity in the form of transaminase elevation and hyperbilirubinaemia has been observed. Additionally, there have been uncommon reports of acute liver failure. Monitoring of hepatic function is recommended in circumstances where Imatinib is combined with chemotherapy regimens also known to be associated with hepatic dysfunction.
Fluid retention :
Occurrences of severe fluid retention (pleural effusion, oedema, pulmonary oedema, ascites, superficial oedema) have been reported in approximately 2.5 % of newly diagnosed CML patients taking Imatinib. Therefore, it is recommended that patients be weighed regularly. An unexpected rapid weight gain should be carefully investigated and if necessary appropriate supportive care and therapeutic measures should be undertaken. In clinical trials, there was an increased incidence of these events in elderly patients and those with a prior history of cardiac disease.
Patients with cardiac disease or renal failure :
Patients with cardiac disease, risk factors for cardiac failure or history of renal failure should be monitored carefully and any patient with signs or symptoms consistent with cardiac or renal failure should be evaluated and treated. In patients with hypereosinophilic syndrome (HES) and cardiac involvement, isolated cases of cardiogenic shock/left ventricular dysfunction have been associated with the initiation of Imatinib therapy. The condition was reported to be reversible with the administration of systemic steroids, circulatory
support measures and temporarily withholding Imatinib. Myelodysplastic (MDS) / myeloproliferative (MPD) diseases and systemic mastocytosis might be associated with high eosinophil levels. Performance of an echocardiogram and determination of serum troponin should therefore be considered in patients with HES/CEL, and in patients with MDS/MPD or SM associated with high eosinophil levels. If either is abnormal, the prophylactic use of systemic steroids (1 to 2 mg/kg) for one to two weeks concomitantly with Imatinib should be considered at the initiation of therapy.
Tumor lysis syndrome :
Cases of tumor lysis syndrome (TLS) have been reported in patients treated with Imatinib. Due to possible occurrence of TLS, correction of clinically significant dehydration and treatment of high uric acid levels are recommended prior to initiation of Imatinib.
Laboratory tests :
Complete blood counts must be performed regularly during therapy with Imatinib. Treatment of CML patients with Imatinib has been associated with neutropenia or thrombocytopenia. However, the occurrence of these cytopenias is dependent on the stage of the disease being treated and they were more frequent in patients with accelerated phase CML or blast crisis as compared to patients with chronic phase CML. Treatment with Imatinib may be interrupted or the dose be reduced, as recommended in section Dosage and administration. Liver function (transaminases, bilirubin, alkaline phosphatase) should be monitored regularly in patients receiving Imatinib. As recommended in Dosage and administration, nonhaematological adverse drug reactions, these laboratory abnormalities
should be managed with interruption and/or dose reduction of the treatment with Imatinib.
Imatinib and its metabolites are not excreted via the kidney to a significant extent. Creatinine clearance (CrCL) is known to decrease with age, and age did not significantly affect Imatinib kinetics. In patients with impaired renal function, imatinib plasma exposure seems to be higher than that in patients with normal renal function, probably due to an elevated plasma level of alpha-acid glycoprotein (AGP), an imatinib-binding protein, in these patients. There is no correlation between imatinib exposure and the degree of renal impairment, as classified by the measurement of creatinine clearance (CrCL), between patients with mild (CrCL : 40 to 59 ml/min) and severe (CrCL : < 20 ml/min) renal impairment. However, as recommended in section Dosage and administration, the starting dose of imatinib can be reduced if not tolerated.
Children and adolescents :
There have been case reports of growth retardation occurring in children and pre-adolescents receiving imatinib. The long term effects of prolonged treatment with Imatinib on growth in children are unknown. Therefore, close monitoring of growth in children under Imatinib treatment is recommended.
Pregnancy : Category D
Studies in animals have shown reproductive toxicity. There are no clinical trials on the use of imatinib in pregnant women. There have been post-market reports of spontaneous abortions and infant congenital anomalies from women who have taken imatinib. Imatinib should be used during pregnancy only if the expected benefit outweighs the potential risk to the foetus. If it is used during pregnancy, the patient must be informed of the potential risk to the foetus.
Nursing Mothers :
Both imatinib and its active metabolite can be distributed into human milk. The milk plasma ratio was determined to be 0.5 for imatinib and 0.9 for the metabolite, suggesting greater distribution of the metabolite into the milk. Considering the combined concentration of imatinib and of the metabolite and the maximum daily milk intake by infants the total exposure would be expected to be low (~10 % of a therapeutic dose). However, since the effects of low-dose exposure of the infant to imatinib are unknown, women taking Imatinib should not breast feed.
Paediatric Use :
The safety and efficacy of imatinib in paediatric patients is not established.
EFFECTS ON ABILITY TO DRIVE AND USE MACHINES :
Reports of motor vehicle accidents have been received in patients receiving Imatinib. While most of these reports are not suspected to be caused by Imatinib, patients should be advised that they may experience undesirable effects such as dizziness, blurred vision or somnolence during treatment with Imatinib. Therefore, caution should be recommended when driving a car or operating machinery.
INTERACTION AND INCOMPATIBILITIES:
Drugs that may alter Imatinib plasma concentrations
Drugs that may increase Imatinib plasma concentrations :
Caution is recommended when administering IMATINIB CAPSULES I.P. with inhibitors of the CYP3A4 family (e.g. ketoconazole, itraconazole, erythromycin, clarithromycin). Substances that inhibit the cytochrome P450 isoenzyme (CYP3A4) activity may decrease metabolism and increase imatinib concentrations. There is a significant increase in exposure to imatinib when IMATINIB CAPSULES I.P. is co-administered with ketoconazole (CYP3A4 inhibitor).
Drugs that may decrease Imatinib plasma concentrations :
Substances that are inducers of CYP3A4 activity may increase metabolism and decrease imatinib plasma concentrations. Co-medications that induce CYP3A4 (e.g., dexamethasone, phenytoin, carbamazepine, rifampicin, phenobarbital or St. John’s Wort) may reduce exposure to IMATINIB CAPSULES I.P. No specific studies have been performed and caution is recommended. Drugs that may have their plasma concentration altered by IMATINIB CAPSULES I.P. Imatinib increases the mean Cmax and AUC of simvastatin (CYP3A4 substrate) 2- and 3.5- fold, respectively, suggesting an inhibition of the CYP3A4 by imatinib. Particular caution is recommended when administering IMATINIB CAPSULES I.P. with CYP3A4 substrates that have a narrow therapeutic window (e.g., cyclosporine or pimozide). IMATINIB CAPSULES I.P. will increase plasma concentration of other CYP3A4 metabolized drugs (e.g., triazolo-benzodiazepines, dihydropyridine calcium channel blockers, certain HMG-CoA reductase inhibitors, etc.) Because warfarin is metabolized by CYP2C9, patients who require anticoagulation should receive low-molecular weight or standard heparin. In vitro, IMATINIB CAPSULES I.P. inhibits the cytochrome P450 isoenzyme CYP2D6 activity at similar concentrations that affect CYP3A4 activity. Systemic exposure to substrates of CYP2D6 is expected to be increased when co-administered with IMATINIB CAPSULES I.P. No specific studies have been performed and caution is recommended.
SIDE EFFECTS :
Cardiovascular :
Fluid retention in up to 70 % of patients, including superficial oedema, pleural effusion. Severe oedema in 1 % to 5 % of patients.
Central Nervous System :
Headache, fatigue, and pyrexia (less than 40 % incidence for each); weakness reported.
Dermatologic :
Skin rash in up to 40 % of patients; night sweats and pruritus.
Gastrointestinal :
Moderate potential for nausea and vomiting; diarrhoea and abdominal pain common; dyspepsia, anorexia, and constipation reported. Elevated LFTs (up to 3.5 %), which resolve within 1 week of dose reduction or discontinuation.
Genitourinary :
Foetal malformations and reduced fertility in rats.
Haematologic :
Neutropenia, thrombocytopaenia , and anaemia common, with white count recovery in 2 to 3 week and platelet recovery in 3 to 4 week. Haemorrhage common (35 % incidence in blast crisis, 48 % in accelerated phase, and 13 % in chronic phase), although severe haemorrhage only.
Metabolic :
Hypokalaemia.
Musculoskeletal :
Muscle cramps or pain common; arthralgia, myalgia.
Respiratory :
Cough, dyspnoea, nasopharyngitis.
OVERDOSAGE AND TREATMENT OF OVEDOSAGE :
Experience with doses greater than 800 mg is limited. In the event of overdosage , the patient should be observed and appropriate supportive treatment given. An oral dose of 1200 mg/m2/day, approximately 2.5 times the human dose of 800 mg, based on body surface area, was not lethal to rats following 14 days of administration. A dose of 3600 mg/m2/day, approximately 7.5 times the human dose of 800 mg, was lethal to rats after 7-10 administrations, due to general deterioration of the animals with secondary degenerative histological changes in many tissues.
STORAGE :
Store below 30°C (86°F), protected from moisture and light.
Do not refrigerate.
SHELF LIFE :
24 months from the date of manufacture.
PRESENTATION :
IMATINIB CAPSULES I.P. contains Imatinib Mesylate I.P. equivalent to Imatinib 400 mg.
3 Strips of 10 Capsules per Box.
IMATINIB CAPSULES I.P. contains Imatinib Mesylate I.P. equivalent to Imatinib 100 mg.
5 Strips of 10 Capsules per Box.
Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.

 Cardiovascular
Cardiovascular





