
100 mg
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
DIMERCAPTOSUCCINIC ACID CAPSULES (Dimercaptosuccinic acid) is an orally active, heavy metal chelating agent.Chemically, Dimercaptosuccinic acid is meso 2,3-dimercaptosuccinic acid (DMSA). The molecular formula is C4H6O4S2 and molecular weight is 182.2.
STRUCTURAL FORMULA :
Its structural formula is :
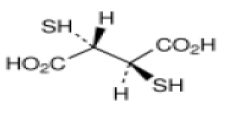
DIMERCAPTOSUCCINIC ACID CAPSULES are white to pale yellow crystalline powder filled in hard gelatin capsule of suitable size.
COMPOSITION :
Each hard gelatin capsule contains :
Dimercaptosuccinc acid 100 mg
Excipients q.s.
Approved colours used in empty capsule shells.
ACTIONS :
Orally active Dimercaptosuccinc acid is a heavy metal chelating agent that forms stable, water-soluble complexes with lead and consequently increases the urinary excretion of lead. The site of lead chelation by Dimercaptosuccinc acid is not known.
Other actions :
Dimercaptosuccinc acid chelates other heavy metals such as arsenic and mercury.
PHARMACOKINETICS :
Dimercaptosuccinc acid rapidly but incompletely absorbed after oral doses. It undergoes rapid and extensive metabolism and is excreted mainly in the urine with small amounts excreted in the bile and via the lungs.
INDICATIONS :
DIMERCAPTOSUCCINIC ACID CAPSULES is indicated for the treatment of lead poisoning in paediatric patients with blood lead levels above 45 mcg/dL. DIMERCAPTOSUCCINIC ACID CAPSULES is not indicated for prophylaxis of lead poisoning in a lead-containing environment; the use of DIMERCAPTOSUCCINIC ACID CAPSULES should always be accompanied by identification and removal of the source of the lead exposure.
Administration :
DIMERCAPTOSUCCINIC ACID CAPSULES are for oral administration.
Dosage :
Start dosage at 10 mg/kg or 350 mg/m² every eight hours for five days. Initiation of therapy at higher doses is not recommended. (See Table for Dosing chart and number of capsules.) Reduce frequency of administration to 10 mg/kg or 350 mg/m² every 12 hours (two-thirds of initial daily dosage) for an additional two weeks of therapy. A course of treatment lasts 19 days. Repeated courses may be necessary if indicated by weekly monitoring of blood lead concentration. A minimum of two weeks between courses is recommended unless blood lead levels indicate the need for more prompt treatment.
In young paediatric patients who cannot swallow capsules, DIMERCAPTOSUCCINIC ACID CAPSULES can be administered by separating the capsule and sprinkling the powder on a small amount of soft food or putting them in a spoon and following with fruit drink.
Identification of the source of lead in the paediatric patient’s environment and its abatement are critical to a successful therapy outcome. Chelation therapy is not a substitute for preventing further exposure to lead and should not be used to permit continued exposure to lead. Patients who have received Edetate calcium disodium with or without BAL may use DIMERCAPTOSUCCINIC ACID CAPSULES for subsequent treatment after an interval of four weeks. Data on the concomitant use of DIMERCAPTOSUCCINIC ACID CAPSULES with Edetate calcium disodium with or without BAL are not available, and such use is not recommended.
CONTRAINDICATIONS :
DIMERCAPTOSUCCINIC ACID CAPSULES should not be administered to patients with a history of allergy to the drug.
Keep out of reach of paediatric patients. DIMERCAPTOSUCCINIC ACID CAPSULES is not a substitute for effective abatement of lead exposure. Mild to moderate neutropenia has been observed in some patients receiving dimercaptosuccinic acid. While a causal relationship to dimercaptosuccinic acid has not been definitely established, neutropenia has been reported with other drugs in the same chemical class. A complete blood count with white blood cell differential and direct platelet counts should be obtained prior to and weekly during treatment with dimercaptosuccinic acid. Therapy should either be withheld or discontinued if the absolute neutrophil count (ANC) is below 1200/mcl and the patient followed closely to document recovery of the ANC to above 1500/mcl or to the patient’s baseline neutrophil count. There is limited experience with reexposure in patients who have developed neutropenia.Therefore, such patients should be rechallenged only if the benefit of dimercaptosuccinic acid therapy clearly outweighs the potential risk of another episode of neutropenia and then only with careful patient monitoring. Patients treated with dimercaptosuccinic acid should be instructed to promptly report any signs of infection. If infection is suspected, the above laboratory tests should be conducted immediately.
PRECAUTIONS :
The extent of clinical experience with DIMERCAPTOSUCCINIC ACID CAPSULES is limited. Therefore, patients should be carefully observed during treatment. Elevated blood lead levels and associated symptoms may return rapidly after discontinuation of DIMERCAPTOSUCCINIC ACID CAPSULES because of redistribution of lead from bone stores to soft tissues and blood. After therapy, patients should be monitored for rebound of blood lead levels, by measuring blood lead levels at least once weekly until stable. However, the severity of lead intoxication (as measured by the initial blood lead level and the rate and degree of rebound of blood lead) should be used as a guide for more frequent blood lead monitoring.
All patients undergoing treatment should be adequately hydrated. Caution should be exercised in using DIMERCAPTOSUCCINIC ACID CAPSULES therapy in patients with compromised renal function. Limited data suggests that DIMERCAPTOSUCCINIC ACID
CAPSULES is dialyzable, but that the lead chelates are not. Transient mild elevations of serum transaminases have been observed in 6-10 % of patients during the course of DIMERCAPTOSUCCINIC ACID CAPSULES therapy. Serum transaminases should be monitored before the start of therapy and at least weekly during therapy. Patients with a history of liver disease should be monitored closely. No data are available regarding the metabolism of dimercaptosuccinic acid in patients with liver disease.
Clinical experience with repeated courses is limited. The safety of uninterrupted dosing longer than three weeks has not been established and it is not recommended. The possibility of allergic or other mucocutaneous reactions to the drug must be borne in mind on readministration (as well as during initial courses). Patients requiring repeated courses of DIMERCAPTOSUCCINIC ACID CAPSULES should be monitored during each treatment course. One patient experienced recurrent mucocutaneous vesicular eruptions of increasing severity affecting the oral mucosa, the external urethral meatus and the perianal area on the third, fourth and fifth courses of the drug. The reaction resolved between courses and upon discontinuation of therapy.
Pregnancy : Category C
DIMERCAPTOSUCCINIC ACID CAPSULES has been shown to be teratogenic and foetotoxic in pregnant mice when given subcutaneously in a dose range of 410 to 1640 mg/kg/day during the period of organogenesis. In a developmental study in rats, DIMERCAPTOSUCCINIC ACID CAPSULES produced maternal toxicity and deaths at the dose of 720 mg/kg/day or more during organogenesis. The dose of 510 mg/kg/day was the highest tolerable dose in pregnant rats. Impaired development of reflexes was noted in pups of 720 mg/kg/day group dam. There are no adequate and well controlled studies in pregnant women. DIMERCAPTOSUCCINIC ACID CAPSULES should be used during pregnancy only if the potential benefit justifies the potential risk to the foetus.
Nursing Mothers :
It is not known whether this drug is excreted in human milk. Because many drugs and heavy metals are excreted in human milk, nursing mothers requiring DIMERCAPTOSUCCINIC ACID CAPSULES therapy should be discouraged from nursing their infants.
Paediatric Use :
Safety and efficacy in paediatric patients less than 12 months of age have not been established.
INTERACTION AND INCOMPATIBILITIES :
DIMERCAPTOSUCCINIC ACID CAPSULES is not known to interact with other drugs including iron supplements; interactions have not been systematically studied. Concomitant administration of DIMERCAPTOSUCCINIC ACID CAPSULES with other chelation therapy, such as Edetate calcium disodium is not recommended.
LABORATORY TESTS INTERACTION :
Dimercaptosuccinic acid may interfere with serum and urinary laboratory tests. In vitro studies have shown DIMERCAPTOSUCCINIC ACID CAPSULES to cause false positive results for ketones in urine using nitroprusside reagents such as Ketostix and falsely decreased measurements of serum uric acid and CPK.
SIDE EFFECTS :
DIMERCAPTOSUCCINIC ACID CAPSULES may cause gastrointestinal disorders, skin rashes, increases in serum transaminase, flu-like symptoms, drowsiness, and dizziness. Mild to moderate neutropenia has been reported in some patients and regular full blood counts are recommended during therapy. DIMERCAPTOSUCCINIC ACID CAPSULES should be used with caution in patients with renal impairment or a history of hepatic disease.
INFORMATION FOR PATIENTS :
Patients should be instructed to maintain adequate fluid intake. If rash occurs, patients should consult their physician. Patients should be instructed to promptly report any indication of infection, which may be a sign of neutropenia. In young paediatric patients unable to swallow capsules, the contents of the capsule can be administered in a small amount of food.
OVERDOSAGE :
Doses of 2300 mg/kg in the rat and 2400 mg/kg in the mouse produced ataxia, convulsions, labored respiration and frequently death. No case of overdosage has been reported in humans. Limited data indicate that DIMERCAPTOSUCCINIC ACID CAPSULES is dialyzable.
TREATMENT OF OVERDOSAGE :
In case of acute overdosage, induction of vomiting or gastric lavage followed by administration of an activated charcoal slurry and appropriate supportive therapy are recommended. Patients in whom intentional overdose is confirmed or suspected should be referred for psychiatric consultation.
STORAGE :
Store below 30°C (86°F), protected from moisture and light.
Do not refrigerate.
Avoid excessive heat.
SHELF LIFE :
24 months from the date of manufacture.
PRESENTATION :
DIMERCAPTOSUCCINIC ACID CAPSULES contains Dimercaptosuccinic acid 100 mg.
1 Bottle of 100 Capsules per Box.
Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.

 Cardiovascular
Cardiovascular





