
300 mg
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
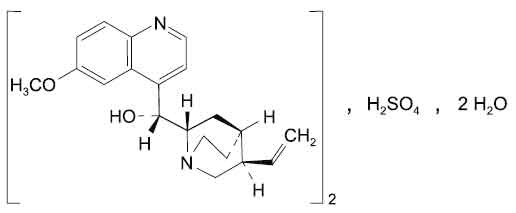
QUININE SULFATE TABLETS B.P. (Quinine Sulfate) is antiprotozoal, antimalarial. Chemically, Quinine Sulfate is bis[(R)-[(2S,4S,5R)-5-ethenyl-1-azabicyclo[2.2.2]oct-2-yl] (6-methoxyquinolin-4-yl)methanol] sulfate. The molecular formula is C40H50N4O8S,2H2O and molecular weight is 783.
STRUCTURAL FORMULA :
Its structural formula is :
QUININE SULFATE TABLETS B.P. are white coloured, circular, biconvex film coated tablets plain on both side.
COMPOSITION :
Each film coated tablet contains :
Quinine Sulfate B.P. 300 mg
Excipients q.s.
Colour : Titanium Dioxide B.P.
ACTIONS :
Quinine is a cinchona alkaloid and a 4-methanolquinoline antimalarial that is a rapid-acting blood schizontocide with activity against Plasmodium falciparum, P. vivax, P. ovale, and P. malariae. It is active against the gametocytes of P. malariae and P. vivax, but not against mature gametocytes of P. falciparum. The precise mechanism of action of quinine is unclear but it may interfere with lysosome function or nucleic acid synthesis in the malaria parasite. Since it has no activity against exoerythrocytic forms, quinine does not produce a radical cure in vivax or ovale malarias. Quinine has effects on the motor end-plate of skeletal muscle and prolongs the refractory period. Like quinidine, quinine is a sodium channel blocker and, therefore, has local anaesthetic, and both anti- and pro-arrhythmic activity.
The precise mechanism of action of quinine is unclear but it may interfere with lysosome function or nucleic acid synthesis in the malaria parasite.
PHARMACOKINETICS :
The pharmacokinetics of quinine are altered significantly by malaria infection, the major effects being reductions in both its apparent volume of distribution and its clearance.
Absorption :
Quinine is rapidly and almost completely absorbed from the GI tract and peak concentrations in the circulation are attained about 1-3 hours after oral administration of the sulphate.
Distribution :
Plasma protein binding is about 70 % in healthy subjects and rises to 90 % or more in patients with malaria. Quinine is widely distributed throughout the body. Concentrations attained in the CSF of patients with cerebral malaria have been reported to be about 2-7 % of those in the plasma.
Metabolism :
Quinine is extensively metabolised in the liver and rapidly excreted mainly in the urine. Estimates of the proportion of unchanged quinine excreted in the urine vary from less than 5 % to 20 %. The pharmacokinetics of quinine are altered significantly by malaria infection, with reductions in both the apparent volume of distribution and clearance.
Elimination :
Excretion is increased in acid urine. The elimination half-life is about 11 hours in healthy subjects but may be prolonged in patients with malaria. Small amounts of quinine also appear in the bile and saliva. Quinine crosses the placenta and is excreted in the breast milk.
INDICATIONS :
QUININE SULFATE TABLETS B. P. is used for the treatment of falciparum malaria or if the infective species is not known or if the infection is mixed. QUININE SULFATE TABLETS B. P. is also used for the treatment of nocturnal leg cramps.
Administration :
QUININE SULFATE TABLETS B. P. are for oral administration only. QUININE SULFATE TABLETS B. P. may be taken with or after meals to minimise gastro-intestinal irritation.
Dosage :
For the treatment of falciparum malaria :
Adult :
600 mg every 8 hours for 5 - 7 days together with or followed by either doxycycline 200 mg once daily for 7 days or clindamycin 450 mg every 8 hours for 7 days. If the parasite is likely to be sensitive, pyrimethamine 75 mg with sulfadoxine 1.5 g may be given as a single dose together with or after a course of quinine.
Children :
Quinine is well tolerated by children although the salts are bitter. The dosage regimen for quinine by mouth for children is : 10 mg/kg (maximum 600 mg) every 8 hours for 7 days together with or followed by Clindamycin 7 - 13 mg/kg (maximum 450 mg) every 8 hours for 7 days or in children over 12 years, doxycycline 200 mg once daily for 7 days or if the parasite is likely to be sensitive, pyrimethamine with sulfadoxine as a single dose : up to 4 years and body-weight over 5 kg, pyrimethamine 12.5 mg with sulfadoxine 250 mg; 5 - 6 years, pyrimethamine 25 mg with sulfadoxine 500 mg; 7 - 9 years, pyrimethamine 37.5 mg with sulfadoxine 750 mg; 10 - 14 years, pyrimethamine 50 mg with sulfadoxine 1 g; 14 - 18 years, pyrimethamine 75 mg with sulfadoxine 1.5 g.
For the treatment of nocturnal leg cramps :
Adult :
QUININE SULFATE TABLETS B. P., 200 - 300 mg at bedtime are effective in reducing the frequency of nocturnal leg cramps by about 25 % in ambulatory patients. It may take up to 4 weeks for improvement to become apparent; if there is benefit, quinine treatment can be continued. Patients should be monitored closely during the early stages for adverse effects as well as for benefit. Treatment should be interrupted at intervals of approximately 3 months to assess the need for further quinine treatment.
CONTRAINDICATIONS :
- History of Blackwater fever
- Cardiac arrhythmias, history of, or QT prolongation
- Glucose-6-phosphate dehydrogenase (G6PD) deficiency
- Known hypersensitivity to quinine or quinidine or any of the excipients in the formulation.
- Hypoglycaemia
- Myasthenia gravis
- History of purpura, thrombocytopenic
- Tinnitus
- Optic neuritis
- Haemoglobinuria
QUININE SULFATE TABLETS B.P. contains lactose which is contra-indicated in patients with galactosaemia, the glucose-galactose malabsorption syndrome, or lactase deficiency.
Quinine should be used with caution in patients with atrial fibrillation or other serious heart disease. Quinine should be avoided in patients with myasthenia gravis as it may aggravate their condition and cause severe respiratory distress and dysphasia. Glucose-6-phosphate dehydrogenase deficient patients with malaria are at increased risk of haemolysis during quinine therapy. Treatment should be monitored in all patients in case signs of resistance develop. Quinine can affect the results of certain urine tests for alkaloids and steroids. It may also interfere with tests for plasma catecholamines as well as slowing the erythrocyte sedimentation rate. Administration of quinine may give rise to cinchonism, which is generally more severe in overdose, but may also occur in normal therapeutic doses. Patients should be warned not to exceed the prescribed dose, because of the possibility of serious, irreversible side effects in overdose. Such symptoms include tinnitus, impaired hearing, headache, nausea and disturbed vision. Patients hypersensitive to quinidine may be hypersensitive to this medication also. Hypersensitivity to quinine may also occur with symptoms of cinchonism together with urticaria, flushing pruritus, rash, fever, angioedema, dyspnoea and asthma.
Use of Mefloquine with quinine sulfate may increase the chance of side-effects :
Concurrent use of mefloquine with quinine may result in an increased incidence of seizures and of electrocardiogram abnormalities, predisposing the patient to arrhythmias, it is recommended that Mefloquine be administered at least 12 hours after the last dose of quinine. Patients taking weekly mefloquine prophylaxis may be found to have mefloquine-resistant malaria that requires treatment with quinine, because mefloquine has a very long half-life (approximately 20 days) it will remain in the body long after the drug has been discontinued. Although there is insufficient information available, it is recommended that if quinine must be given that the patient be hospitalized, if possible, and monitored for QT prolongation and possible rhythm disturbances. Seizure activity may also be potentiated in these patients. In patients considered to be at high risk for a seizure, additional precautions and interventions may be indicated. Quinine should not be withheld from pregnant women who have life threatening malaria. Treatment with quinine should be monitored in case signs of resistance develop. Quinine may cause unpredictable serious and life-threatening thrombocytopenia, which is thought to be an idiosyncratic hypersensitivity reaction. Quinine should not be prescribed or administered to patients who have previously experienced any adverse reaction to quinine, including that in tonic water or other beverages. Patients should be instructed to stop treatment and consult a physician if signs of thrombocytopenia such as unexplained bruising or bleeding occur. QUININE SULFATE TABLETS B.P. should be used cautiously in diabetic patients.
Pregnancy : Category X
Quinine crosses the placenta; one study found the cord plasma concentration to be approximately one third the concentration of quinine in maternal plasma. Quinine has been used to treat patients with Plasmodium falciparum malaria in the third trimester of pregnancy.
However, the risk of quinine to the foetus must be balanced against the danger of P. falciparum malaria, which is potentially life-threatening, especially during pregnancy. Studies in humans have shown that quinine causes congenital malformations, especially when given in large doses (e.g., up to 30 grams for attempted abortion). These malformations include deafness related to auditory nerve hypoplasia, limb anomalies, visceral defects, and visual changes. In addition, quinine may have an oxytoxic action on the uterus and has been shown to cause abortion when taken in toxic amounts. Stillbirths have also been reported in mothers taking quinine during pregnancy. Studies in rabbits and guinea pigs have shown that quinine is teratogenic. However, no teratogenic effects were seen in mice, rats, dogs, or monkeys.
Nursing mothers :
Quinine sulfate is excreted in breast milk, but no problems in humans have been reported. However, quinine sulfate should not be given to nursing mothers unless the benefits outweigh the risks.
Paediatric Use :
Antimalarial studies performed to date have shown that children have a decreased elimination half-life and volume of distribution; however, paediatrics-specific problems that would limit the usefulness of quinine in children have not been documented.
EFFECTS ON ABILITY TO DRIVE AND USE MACHINES :
QUININE SULFATE TABLETS B.P. may cause visual disturbances and vertigo, hence patients should be advised that if affected they should not drive or operate machinery.
INTERACTIONS AND INCOMPATIBILITIES :
Effect of other drugs on Quinine :
Quinine is metabolised via hepatic oxidative cytochrome P450 pathways, predominantly by CYP3A4. There is the potential for increased Quinine toxicity with concurrent use of potent CYP3A4 inhibitors, which include azole antifungal drugs and HIV protease inhibitors. Sub-optimal Quinine serum levels may result from concomitant use of CYP3A4 inducers, which include rifampicin, barbiturates, carbamazepine and phenytoin. Care should be taken when Quinine is used in combination with other CYP3A4 substrates, especially those causing prolongation of the QT interval.
Effect of Quinine on other drugs :
The plasma concentration of flecainide, digoxin and mefloquine may be increased. Amantadine : Quinine can reduce the renal clearance of amantadine. Cyclosporin : Quinine can decrease serum plasma concentrations of cyclosporin. Cardiac glycosides : Quinine increases plasma concentrations of cardiac glycosides and reduced dosage of concomitant cardiac glycosides such as digoxin to half the maintenance dose may be necessary.
Other drug interactions :
There is an increased risk of ventricular arrhythmias with other drugs which prolong the QT interval, including amiodarone, moxifloxacin, pimozide, thioridazine and halofantrine. Anti-arrhythmics : Concomitant use of amiodarone should be avoided due to the increased risk of ventricular arrhythmias. The plasma concentration of flecainide is increased by quinine. Concomitant use of quinidine may increase the possibility of cinchonism. Antibacterials : There is an increased risk of ventricular arrhythmias when moxifloxacin is given with quinine. Rifampicin can reduced the serum levels of quinine, therefore reducing its therapeutic effect. Anticoagulants : Quinine may cause hypoprothrombinaemia and enhance the effects of anticoagulants. Antihistamines : Concomitant use of terfenadine should be avoided due to the increased risk of ventricular arrhythmias. Antimalarials : According to the manufacturer of artemether with lumefantrine concomitant use should be avoided. There is an increased risk of convulsions when given with mefloquine. Chloroquine and quinine appear to be antagonistic when given together for P falciparum malaria. There is a decrease in plasma concentrations of primaquine. Antipsychotics : There is an increased risk of ventricular arrhythmias and concomitant use should be avoided with pimozide or thioridazine. Hypoglycaemics : There is an increased risk of hypoglycaemia when taken concurrently. Suxamethonium : Quinine enhances the neuromuscular effects of suxamethonium. Ulcer-healing drugs : Cimetidine inhibits quinine metabolism leading to increased plasma-quinine concentrations.
SIDE EFFECTS :
Cinchonism is more common in overdose, but may occur even after normal doses of quinine. In its mild form symptoms include tinnitus, impaired hearing, rashes, headache, nausea and disturbed vision. Its more severe manifestations symptoms may include gastro-intestinal symptoms, oculotoxicity, CNS disturbances, cardiotoxicity and death. Visual disorders may include blurred vision, defective colour perception, visual field constriction and total blindness.
Blood and the lymphatic system disorders :
Thrombocytopenia, intravascular coagulation, hypoprothrombinaemia, haemoglobinuria, oliguria, haemolytic-uraemic syndrome, pancytopenia, haemolysis agranulocytosis and thrombocytopenic purpura have all been reported.
Immune system disorders :
Reports have been received of eczematous dermatitis, oedema, erythema and lichen planus. Hypersensitivity reactions such as asthma, angioneurotic oedema, photosensitivity, hot and flushed skin, fever, pruritis, thrombocytopenic purpura and urticaria have also been reported.
Metabolism and nutrition disorders :
Hypoglycaemia may occur after oral administration although it is more common after parenteral administration.
Psychiatric disorders :
Agitation, confusion.
Nervous system disorders :
Reports of headache, vertigo, excitement, loss of consciousness, coma and death have been received.
Eye disorders :
Blurred vision, defective colour perception, visual field constriction.
Ear and labyrinth disorders :
Tinnitus, impaired hearing.
Cardiac disorders:
There may be atrioventricular conduction disturbances, a fall in blood pressure coupled with a feeble pulse. Prolongation of the QT interval, widening of the QRS complex and T wave flattening has been noted with therapeutic doses.
Respiratory, thoracic and mediastinal disorders :
Bronchospasm, dyspnoea may occur.
Gastro-intestinal disorders :
Diarrhoea, nausea, vomiting and abdominal pain may occur after long term administration of quinine.
Skin and subcutaneous tissue disorders :
Flushing, rash, urticaria, eczematous dermatitis, oedema, erythema, lichen planus, pruritis, photosensitivity.
Musculoskeletal, connective tissue and bone disorders :
Muscle weakness may occur, aggrevation of Myasthenia gravis.
Renal and urinary disorders :
Renal insufficiency and acute renal failure may be due to an immune mechanism or to circulatory failure.
Reproductive system and breast disorders :
Toxic doses of quinine may induce abortion, but it is unwise to withhold the drug if less toxic antimalarials are not available.
OVERDOSAGE :
Quinine overdosage may lead to serious and irreversible side effects and can be fatal. In acute overdosage, symptoms of cinchonism may occur, including convulsions, nausea, vomiting, tinnitus, deafness, headache, vasodilatation and disturbed vision. The visual disorders may be severe and there may be impaired consciousness, coma, respiratory depression, arrhythmia and cardiogenic shock. Fatalities have been reported in adults after doses of 2-8 g. High doses of quinine are teratogenic and may cause miscarriage.
The effects of oculotoxicity may include blurred vision, defective colour perception, visual field constriction and total blindness. The onset of symptoms may vary from a few hours to a day or more after ingestion. Visual disturbances are usually slowly reversible but there may be residual damage. The effects associated with cardiovascular toxicity include conduction abnormalities, ventricular dysrhythmias, anginal symptoms and hypotension leading to cardiac arrest and circulatory failure. Hypokalaemia and hypoglycaemia may also occur.
TREATMENT OF OVERDOSAGE :
Quinine is rapidly absorbed. In patients who present within one hour of ingestion an overdose gastric lavage may be considered, but the risk of aspiration must be considered, especially if there is CNS depression or drowsiness. Multiple doses of activated charcoal may be considered. Other treatment is mostly symptomatic to maintain blood pressure, respiration, renal function and treating arrhythmia, convulsions, hypoglycaemia and acidosis.
STORAGE :
Store below 30°C (86°F), protect from moisture and light.
Do not refrigerate.
SHELF LIFE :
24 months from the date of manufacture.
PRESENTATION :
QUININE SULFATE TABLETS B.P. contains Quinine Sulfate B.P. 300 mg.
10 Blisters of 10 Tablets per Box.
Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.

 Cardiovascular
Cardiovascular





