200 mg/20 ml
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
Zidovudine formerly called azidothymidine (AZT), is a pyrimidine nucleoside analogue active against human immunodeficiency virus (HIV). Chemically, Zidovudine is 3-azido-3-deoxythymidine. The molecular formula is C10H13N5O4 and the molecular weight is 267.24.
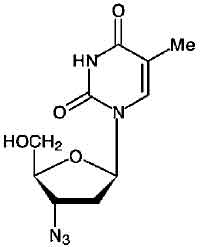
STRUCTURAL FORMULA :
Its structural formula is :
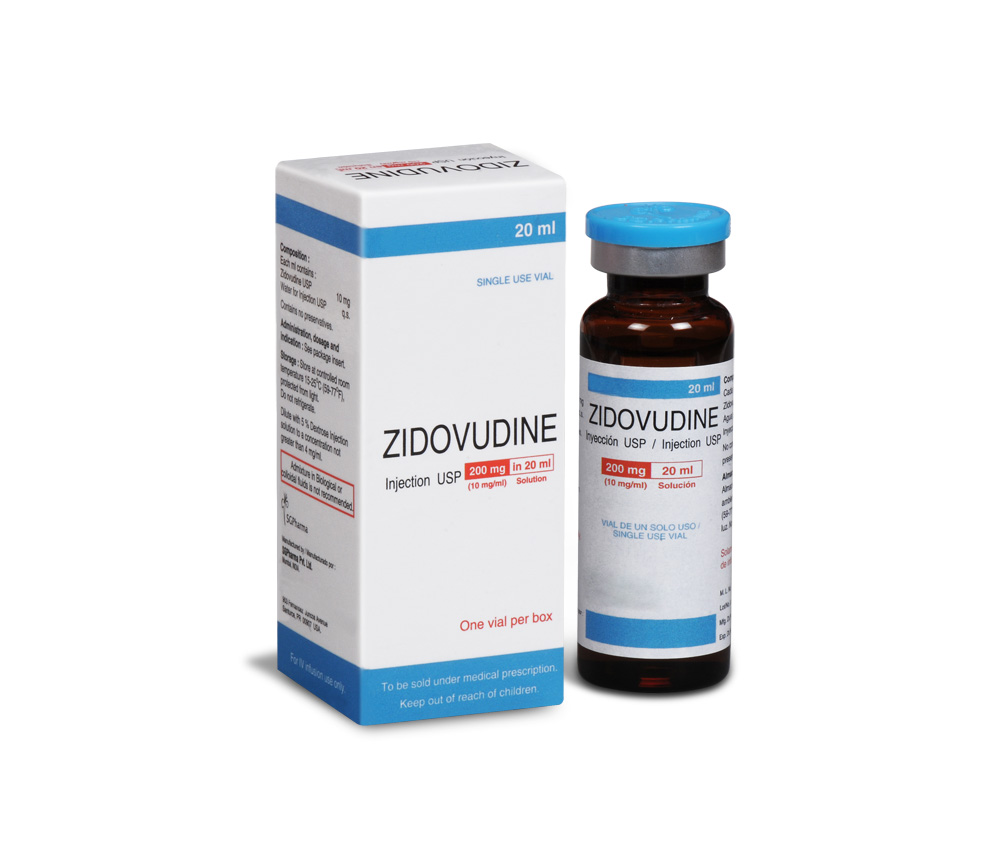
Zidovudine Injection is a clear colourless solution filled in 20 ml amber glass vial.
COMPOSITION :
Each ml contains :
Zidovudine USP 10 mg
Water for Injection USP q.s.
Contains No Preservatives.
ACTIONS :
Zidovudine is a synthetic nucleoside analogue of the naturally occurring nucleoside, thymidine, in which the 3-hydroxy (-OH) group is replaced by an azido (-N3) group. Within cells, Zidovudine is converted to the active metabolite, zidovudine 5-triphosphate (AztTP), by the sequential action of the cellular enzymes. Zidovudine 5-triphosphate inhibits the activity of the HIV reverse transcriptase both by competing for utilization with the natural substrate, deoxythymidine 5-triphosphate (dTTP) and by its incorporation into viral DNA. The lack of a 3-OH group in the incorporated nucleoside analogue prevents the formation of the 5 to 3 phosphodiester linkage essential for DNA chain elongation and therefore, the viral DNA growth is terminated. The active metabolite AztTP is also a weak inhibitor of the cellular DNA polymerase-alpha and mitochondrial polymerase-gamma and has been reported to be incorporated into the DNA of cells in culture.
PHARMACOKINETICS :
Absorption, Distribution and Elimination :
Zidovudine is absorbed rapidly from the gastrointestinal tract, with peak serum levels achieved within about one hour. Pharmacokinetic studies of Zidovudine Injection following intravenous dosing in adults indicate dose-independent kinetics over the range of 1 to 5 mg/kg with a mean Zidovudine half-life of 1.1 hours. The plasma half-life of the prodrug is considerably shorter than the intracellular half-life of active zidovudine 5-triphosphate. Importantly, plasma Zidovudine concentrations do not correlate with intracellular triphosphate concentrations or clinical efficacy. The rate-limiting step in intracellular activation is conversion to the monophosphate. Therefore, higher plasma concentrations of Zidovudine do not proportionately increase intracellular triphosphate concentrations. Zidovudine crosses the blood-brain barrier relatively well and achieves a cerebrospinal fluid (CSF)-to-plasma ratio of approximately 0.6. Zidovudine also is detectable in breast milk, semen and foetal tissue. Zidovudine undergoes rapid first-pass hepatic metabolism by conversion to 5-glucuronyl zidovudine . This metabolite has an elimination half-life of 1 hour. Total urinary recovery of Zidovudine and its major metabolite is approximately 90 %. It is also metabolized in the liver mainly to the inactive glucuronide and is excreted in the urine as unchanged drug and metabolite. As there is some tubular secretion, probenecid can delay excretion. The pharmacokinetics of Zidovudine generally is unaffected by pregnancy and drug concentrations in the newborn approach those of the mother.
Neonates :
The pharmacokinetics of Zidovudine in infants more than 14 days old are reported to be similar to those in adults. Following maternal administration the half-life of Zidovudine in 7 neonates has been reported to be extended to a mean of 13 hours. In infants receiving Zidovudine, those under 14 days of age had lower total clearance, longer terminal half-life (about 3 hours) and higher bioavailability than older infants. Zidovudine clearance was low and the half-life prolonged to about 7 hours in premature infants. The pharmacokinetics of Zidovudine in paediatrics patients greater than 3 months of age is similar to that of Zidovudine in adult patients.
INDICATIONS :
Zidovudine Injection is indicated for the treatment of HIV infection when antiretroviral therapy is warranted. The duration of clinical benefit from antiretroviral therapy may be limited. Alterations in antiretroviral therapy should be considered if disease progression occurs during treatment. Therapy with Zidovudine Injection has been shown to prolong survival and decrease the incidence of opportunistic infections in patients with advanced HIV disease at the initiation of therapy and to delay disease progression in asymptomatic HIV-infected patients. Zidovudine Injection in combination with certain antiretroviral agents has been shown to be superior to monotherapy in one or more of the following : delaying death, delaying development of AIDS, increasing CD4 cell counts and decreasing plasma HIV-1-RNA. Use of Zidovudine Injection in some combinations is based on surrogate marker data. The complete prescribing information for each drug should be consulted before combination therapy which includes Zidovudine Injection is initiated.
Maternal-Foetal HIV Transmission :
Zidovudine Injection is also indicated for the prevention of maternal-foetal HIV transmission as part of a regimen that includes oral Zidovudine beginning between 14 and 34 weeks of gestation, intravenous Zidovudine during labour and administration of Zidovudine Syrup to the newborn after birth. However, transmission to infants may still occur in some cases despite the use of this regimen. The efficacy of this regimen for preventing HIV transmission in women who have received Zidovudine for a prolonged period before pregnancy has not been evaluated. Using this regimen, the relative reduction in transmission risk was found to be 67.5 %. The safety of Zidovudine Injection for the mother or foetus during the first trimester of pregnancy has not been assessed.
Zidovudine Injection is administered intravenously at a constant rate over 1 hour. Rapid infusion or bolus injection should be avoided. Zidovudine Injection should not be given intramuscularly.
FOR THE TREATMENT OF HIV INFECTION :
Adults :
The recommended intravenous dose is 1 mg/kg infused over 1 hour. This dose should be administered 5 to 6 times daily (5 to 6 mg/kg daily). Patients should receive Zidovudine Injection only until oral therapy can be administered. The intravenous dosing regimen equivalent to the oral administration of 100 mg every 4 hours is approximately 1 mg/kg intravenously every 4 hours. The effectiveness of the intravenous dose compared to higher dosing regimens in improving the neurological dysfunction associated with HIV disease is unknown. A small randomized study has found a greater effect of higher doses of Zidovudine Injection on improvement of neurological symptoms in patients with pre-existing neurological disease.
Children :
The recommended dose of Zidovudine Injection in children 3 months to 12 years of age is 120 mg/m2 every 6 hours, infused over 1 hour (480 mg/m2 per day). Do not exceed 160 mg of any individual dose.
PREVENTION OF MATERNAL-FOETAL HIV TRANSMISSION :
The recommended dosing regimen for administration to pregnant women (> 14 weeks of pregnancy) and their newborn infants is :
Maternal dosing :
100 mg orally 5 times per day until the start of labour. During labour and delivery, intravenous Zidovudine should be administered at 2 mg/kg (total body weight) over 1 hour followed by a continuous intravenous infusion of 1 mg/kg/ hour (total body weight) until clamping of the umbilical cord.
New born infants :
2 mg/kg orally every 6 hours starting within 12 hours after birth and continuing through 6 weeks of age. Infants unable to receive Zidovudine Syrup may be administered Zidovudine intravenously at 1.5 mg/kg of body weight infused over 30 minutes, every 6 hours.
Monitoring of patients :
Hematologic toxicities appear to be related to pretreatment bone marrow reserve and to dose and duration of therapy. In patients with poor bone marrow reserve, particularly in patients with advanced symptomatic HIV disease, frequent monitoring of hematological indices is recommended to detect serious anaemia or granulocytopenia. In patients who experience hematologic toxicity, reduction in hemoglobin may occur as early as 2 to 4 weeks and neutropenia usually occurs after 6 to 8 weeks. Patients treated with Zidovudine should be under close clinical observation to manage potential opportunistic infections associated with HIV disease. Prompt recognition of infection or toxicities and appropriate management is required.
DOSE ADJUSTMENT :
Anaemia :
Significant anaemia (hemoglobin of < 7.5 g/dL or reduction of > 25 % of baseline) and/or significant neutropenia (granulocyte count of < 750 cells/mm3 or reduction of > 50 % from baseline) may require a dose interruption until evidence of marrow recovery is observed. In patients who develop significant anaemia, dose interruption does not necessarily eliminate the need for transfusion. For less severe anaemia or neutropenia, a reduction in daily dose may be adequate. If marrow recovery occurs following dose interruption, resumption in dose may be appropriate using adjunctive measures such as epoetin alfa at recommended doses, depending on hematological indices such as serum erythropoetin level and patient tolerance. For patients experiencing pronounced anaemia while receiving chronic co administration of Zidovudine and some of the drugs (e.g. Fluconazole, Valproic acid), Zidovudine dose reduction may be considered.
End-Stage Renal Disease :
In patients maintained on hemodialysis or peritoneal dialysis (CrCl < 15 mL/min), recommended dosing is 1 mg/kg every 6 to 8 hours.
Hepatic Impairment :
There are insufficient data to recommend dose adjustment of Zidovudine Injection in patients with mild to moderate impaired hepatic function or liver cirrhosis. Since Zidovudine Injection is primarily eliminated by hepatic metabolism, a reduction in the daily dose may be necessary in these patients. Frequent monitoring of hematologic toxicities is advised.
METHOD OF PREPARATION :
Zidovudine Injection must be diluted prior to administration. The calculated dose should be removed from 20-mL vial and added to 5 % Dextrose Injection solution to achieve a concentration no greater than 4 mg/mL. Zidovudine Injection does not contain preservatives.
Unused portion of the vial should be discarded.
Recommended Diluents :
5 % Dextrose Injection.
0.9 % Sodium Chloride Injection.
5 % Dextrose Injection and 0.45 % Sodium Chloride Injection.
Lactated Ringers Injection.
5 % Dextrose and Lactated Ringers Injection.
The diluted solution should be administered within 8 hours if stored at 25°C (77°F) or 24 hours if refrigerated at 2 - 8°C (35.6 - 46.4°F) to minimize potential administration of a microbially contaminated solution.
CONTRAINDICATIONS :
Zidovudine Injection is contraindicated for patients who have potentially life-threatening allergic reactions to any of the components of the formulations.
WARNINGS :
Zidovudine should not be administered concomitantly with combination products of Zidovudine. The incidence of adverse reactions appears to increase with disease progression; patients should be monitored carefully, especially as disease progression occurs.
Bone Marrow Suppression :
Zidovudine should be used with extreme caution in patients who have bone marrow compromise evidenced by granulocyte count < 1000 cells/mm3 or hemoglobin < 9.5 g/ dL. In patients with advanced symptomatic HIV disease, anaemia and neutropenia were the most significant adverse events observed. There have been reports of pancytopenia associated with the use of Zidovudine, which was reversible in most instances, after discontinuance of the drug. However, significant anaemia, in many cases requiring dose adjustment, discontinuation of Zidovudine and/or blood transfusions, has occurred during treatment with Zidovudine alone or in combination with other antiretrovirals. Frequent blood counts are strongly recommended in patients with advanced HIV disease who are treated with Zidovudine. For HIV-infected individuals and patients with asymptomatic or early HIV disease, periodic blood counts are recommended. If anaemia or neutropenia develops, dosage adjustments may be necessary.
Myopathy :
Myopathy and myositis with pathological changes similar to that produced by HIV disease have been associated with prolonged use of Zidovudine.
Lactic Acidosis/Severe Hepatomegaly with Steatosis :
Rare occurrences of potentially fatal lactic acidosis in the absence of hypoxemia and severe hepatomegaly with steatosis have been reported with the use of certain antiretroviral nucleoside analogues, including Zidovudine Injection and Zalcitabine. Lactic acidosis should be considered whenever a patient receiving therapy with Zidovudine develops unexplained tachypnea, dyspnea, or fall in serum bicarbonate level. Under these circumstances, therapy with Zidovudine should be suspended until the diagnosis of lactic acidosis has been excluded. Caution should be exercised when administering Zidovudine to any patient, particularly obese women, with hepatomegaly, hepatitis, or other known risk factors for liver disease. These patients should be followed closely while on therapy with Zidovudine. Treatment with Zidovudine should be suspended in the setting of rapidly elevating aminotransferase levels, progressive hepatomegaly, or metabolic/lactic acidosis of unknown etiology.
Other Serious Adverse Reactions :
Reports of pancreatitis, sensitization reactions (including anaphylaxis in one patient), vasculitis and seizures have been rare. These adverse events, except for sensitization, have also been associated with HIV disease. Changes in skin and nail pigmentation have been associated with the use of Zidovudine. Co administration of Zidovudine with other drugs metabolized by glucuronidation should be avoided because the toxicity of either drug may be potentiated. Before combination therapy with Zidovudine is initiated, consult the complete prescribing information for each drug.
PRECAUTIONS :
General :
Zidovudine is eliminated from the body primarily by renal excretion following metabolism in the liver (glucuronidation). In patients with severely impaired renal function, dosage reduction is recommended. Although the data are limited, Zidovudine concentrations appear to be increased in patients with severely impaired hepatic function, which may increase the risk of hematologic toxicity. Very rare occurrences of pure red cell aplasia have been reported with Zidovudine use. Discontinuation of Zidovudine has resulted in normalization of hematological parameters in patients with suspected Zidovudine-induced pure red cell aplasia.
Fat Redistribution :
Redistribution/accumulation of body fat including central obesity, dorsocervical fat enlargement ("buffalo hump"), peripheral wasting, facial wasting, breast enlargement and "cushingoid appearance" have been observed in patients receiving antiretroviral therapy.
Use in Infancy :
A positive test for HIV-antibody in children under 15 months of age may represent passively acquired maternal antibodies, rather than an active antibody response to infection in the infant. Thus, the presence of HIV-antibody in a child less than 15 months of age must be interpreted with caution, especially in the asymptomatic infant.
Use in Children :
See INDICATIONS, SIDE EFFECTS and DOSAGE AND ADMINISTRATION sections. The pharmacokinetics of Zidovudine in paediatrics patients greater than 3 months of age is similar to that of Zidovudine in adult patients.
Pregnancy Category C :
Pregnant women considering the use of Zidovudine during pregnancy for prevention of HIV-transmission to their infants should be advised that transmission may still occur in some cases despite therapy. The long-term consequences of in utero and infant exposure to Zidovudine are unknown.
Nursing Mothers :
The Centers for Disease Control and Prevention recommend that HIV-infected mothers not breastfeed their infants to avoid risking postnatal transmission of HIV. Zidovudine is excreted in human milk. Because of both the potential for HIV transmission and the potential for serious adverse reactions in nursing infants, mothers should be instructed not to breastfeed if they are receiving Zidovudine.
INTERACTIONS AND INCOMPATIBILITIES :
For patients experiencing pronounced anaemia or other severe Zidovudine-associated events while receiving chronic administration of Zidovudine and some of the following drugs : Atovaquone, Fluconazole, Methadone, Nelfinavir, Probenecid, Rifampin, Ritonavir, Valproic acid - Zidovudine dose reduction may be considered.
Doxorubicin :
Concomitant use of Zidovudine with Doxorubicin should be avoided since an antagonistic relationship has been demonstrated in vitro.
Antiretroviral Agents :
Concomitant use of Zidovudine with Stavudine should be avoided since an antagonistic relationship has been demonstrated in vitro. Some nucleoside analogues affecting DNA replication, such as Ribavirin, antagonize the in vitro antiviral activity of Zidovudine against HIV; concomitant use of such drugs should be avoided.
Phenytoin :
Phenytoin plasma levels have been reported to be low in some patients receiving Zidovudine. In one study, a 30 % decrease in oral Zidovudine clearance was observed with Phenytoin.
Probenecid :
Limited data suggest that Probenecid may increase Zidovudine levels by inhibiting glucuronidation and/or reducing renal excretion of Zidovudine. Some patients who have used Zidovudine concomitantly with Probenecid have developed flu-like symptoms consisting of myalgia, malaise, and/or fever and maculopapular rash.
Fluconazole :
The co administration of Fluconazole with Zidovudine has been reported to interfere with the oral clearance and metabolism of Zidovudine. The clinical significance of the interaction is unknown.
Bone Marrow Suppressive Agents / Cytotoxic Agents / Interferon-alpha / Ganciclovir :
Co administration of Zidovudine with drugs that are cytotoxic or which interfere with RBC/WBC number or function (e.g. Dapsone, Flucytosine, Vincristine, Vinblastine or Adriamycin or with Interferon-alpha or Ganciclovir) may increase the risk of hematologic toxicity.
Other Agents :
There is one published report of neurotoxicity (profound lethargy) associated with concomitant use of Zidovudine and Acyclovir.
Incompatibility :
Admixture in biologic or colloidal fluids (e.g. blood products, protein solutions, etc.) is not recommended.
SIDE EFFECTS :
The adverse events reported during intravenous administration of Zidovudine Injection are similar to those reported with oral administration; neutropenia and anaemia were reported most frequently. Long-term intravenous administration beyond 2 to 4 weeks has not been studied in adults and may enhance hematologic adverse events. Local reaction, pain and slight irritation during intravenous administration occur infrequently.
Monotherapy :
Adults :
The frequency and severity of adverse events associated with the use of Zidovudine in adults are greater in patients with more advanced infection at the time of initiation of therapy. The following are events reported at a statistically significant greater incidence for patients receiving
Zidovudine in a monotherapy study :
Body as a whole : Asthenia, headache, malaise. Gastrointestinal : Anorexia, constipation, nausea, vomiting. In addition to the adverse events listed above, other adverse events observed in clinical studies were abdominal cramps, abdominal pain, arthralgia, chills, dyspepsia, fatigue, hyperbilirubinaemia, insomnia, musculoskeletal pain, myalgia and neuropathy. Selected laboratory abnormalities observed during a clinical study of monotherapy with Zidovudine were anaemia, granulocytopenia and elevated ALT/AST.
Paediatrics :
Selected clinical adverse events and physical findings with a > 5 % frequency during therapy with Lamivudine 4 mg/kg twice daily plus Zidovudine 160 mg/m2 3 times daily compared with Didanosine in therapy-naive (< 56 days of antiretroviral therapy) paediatric patients are listed below.
Body as a whole : Fever.
Digestive : Hepatomegaly, nausea & vomiting, diarrhoea, stomatitis, splenomegaly.
Respiratory : Cough, abnormal breath sounds/wheezing.
Ear, Nose and Throat : Signs or symptoms of ears, nasal discharge or congestion.
Other : Skin rashes, lymphadenopathy.
Selected laboratory abnormalities experienced by therapy-naive (< 56 days of antiretroviral therapy) paediatric
patients are : Neutropenia (ANC < 400 cells/mm3), Anaemia (Hgb < 7.0 g/dL), Thrombocytopenia (platelets < 50,000/mm3 ), ALT (> 10 x ULN), AST (> 10 x ULN),Lipase (> 2.5 x ULN),Total amylase (> 2.5 x ULN).
Additional adverse events reported in open-label studies in paediatrics patients receiving Zidovudine 180 mg/m2 every 6 hours were congestive heart failure, decreased reflexes, ECG abnormality, oedema, haematuria, left ventricular dilation, macrocytosis, nervousness/irritability and weight loss. The clinical adverse events reported among adult recipients of Zidovudine may also occur in paediatric patients.
Observed during Clinical Practice :
In addition to adverse events reported from clinical trials, the following events have been identified during use of Zidovudine in clinical practice. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. These events have been chosen for inclusion due to either their seriousness, frequency of reporting, potential causal connection to Zidovudine, or a combination of these factors. Body as a whole : Back pain, chest pain, flu-like syndrome, generalized pain, redistribution/accumulation of body fat.
Cardiovascular : Cardiomyopathy, syncope.
Gastrointestinal : Constipation, dysphagia, flatulence, oral mucosa pigmentation, mouth ulcer.
General : Sensitisation reactions including anaphylaxis and angioedema, vasculitis.
Haemic and Lymphatic : Aplastic anaemia, haemolytic anaemia, leucopenia, lymphadenopathy, pancytopenia with marrow hypoplasia, pure and red cell aplasia.
Hepatobiliary Tract and Pancreas : Hepatitis hepatomegaly with steatosis, jaundice, lactic acidosis, pancreatitis.
Musculoskeletal : Increased CPK, increased LDH, muscle spasm, myopathy and myositis with pathological changes (similar to that produced by HIV disease), rhabdomyolysis, tremor.
Nervous : Anxiety, confusion, depression, dizziness, loss of mental acuity, mania, paraesthesia, seizures,somnolence, vertigo.
Respiratory : Cough, dyspnoea, rhinitis, sinusitis.
Skin : Changes in skin and nail pigmentation, pruritus, rash, Stevens-Johnson syndrome, toxic epidermal necrolysis, sweat, urticaria.
Special Senses : Amblyopia, hearing loss, photophobia, taste perversion.
INFORMATION FOR PATIENTS :
Zidovudine Injection is prescribed to slow down the effects of the HIV virus; it is not a cure. Illnesses associated with HIV infection, including other infections, may still happen. Therefore, it is very important to keep appointments with your doctor and to report any change in your health as it occurs. Contact your doctor if you experience muscle weakness, shortness of breath, symptoms of hepatitis or pancreatitis. Inform your doctor about all the drugs you are taking & ask if you decide to take any new drugs, even if these are available without a prescription. Altering the dose without the direct advice of your physician is unwise. HIV is usually spread by sexual contact or by contaminated needles. This risk still exists during Zidovudine therapy; thus, the practice of safe sex and avoidance of sharing needles is imperative.
OVERDOSAGES :
Cases of acute overdoses in both paediatric patients and adults have been reported with doses upto 50 grams. None were fatal. The only consistent finding in these cases of overdose was spontaneous or induced nausea and vomiting. Hematologic changes were transient and not severe. Some patients experienced nonspecific CNS symptoms such as headache, dizziness, drowsiness, lethargy and confusion. One report of a grand mal seizure possibly attributable to Zidovudine occurred in a 35 year old male 3 hours after ingesting 36 grams of Zidovudine. No other cause could be identified. All patients recovered without permanent squelae. Hemodialysis appears to have a negligible effect on the removal of Zidovudine while elimination of its primary metabolite, GZDV is enhanced.
PHARMACEUTICAL PRECAUTIONS :
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit. If either of them is observed, the solution should be discarded.
STORAGE :
Store at controlled room temperature 15-25°C (59-77°F), protected from light.
Do not refrigerate.
SHELF LIFE :
24 months from the date of manufacture.
PRESENTATION :
Single use 20 ml Amber Tubular vial of 10mg/ml, each in a Box.
Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.

 Cardiovascular
Cardiovascular