250 mcg/ml
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
TETRACOSACTIDE INJECTION b.p. (Tetracosactide) is a corticotropic peptide. Chemically Tetracosactide is sequence of amino acids which is same as that of the first 24 residues of human corticotrophin. The molecular formula is C136H210N40O31S and molecular weight is 2933.
STRUCTURAL FORMULA :
Its structural formula is :
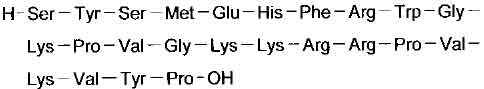
TETRACOSACTIDE INJECTION b.p. is a sterile, clear, colourless solution filled in amber ampoule of suitable size.
COMPOSITION :
Each ml contains :
Tetracosactide B.P. 250 mcg
(as acetate)
Water for Injections B.P. q.s.
ACTIONS :
Tetracosactide acetate consists of the first 24 amino acids occurring in the ACTH sequence and displays the same physiological properties as ACTH. In the adrenal cortex, it stimulates the biosynthesis of glucocorticoids, mineralocorticoids, and, to a lesser extent androgens. The site of action of ACTH is the plasma membrane of the adrenocortical cells, where it binds to a specific receptor. The hormone-receptor complex activates adenylate cyclase, stimulating the production of cyclic AMP (adenosine monophosphate) and so promoting the synthesis of pregnenolone from cholesterol. From pregnenolone the various corticosteroids are produced via different enzymatic pathways.
PHARMACOKINETICS :
Absorption
Tetracosactide is rapidly absorbed from the I.M. injection site.
Distribution
Tetracosactide is rapidly distributed and concentrated in the adrenals and kidneys, which lead to rapid decrease in its plasma levels. There is no evidence of binding of ACTH to any particular plasma protein. Tetracosactide has an apparent distribution volume of about 0.4 l/kg. Tetracosactide apparently does not cross the placenta and it is unknown whether tetracosactide passes into the breast milk.
Biotransformation / Metabolism
In serum, tetracosactide is rapidly degraded by enzymatic hydrolysis, first to inactive oligopeptides, then to free amino acids. Its rapid elimination from plasma is probably attributable not so much to this relatively slow process as to the fact that the active substance is rapidly concentrated in the adrenals and kidneys.
Elimination
The plasma elimination half-life following I.V. injection is about 7 minutes in the first hour (first phase), about 37 minutes in the next hour (second phase), and thereafter about 3 hours (terminal phase).
Following an intravenous dose of 131I-labelled beta-24-corticotrophin, 95 to 100 % of the radioactivity is excreted in the urine within 24 hours.
Clinical studies
No recent clinical trial was conducted with TETRACOSACTIDE INJECTION b.p. I.M./I.V.
Non-clinical safety data
No studies have been performed to evaluate the mutagenic or carcinogenic potential of tetracosactide. No standard animal studies on fertility and reproduction toxicity have been performed with tetracosactide.
INDICATIONS :
Diagnostic test for the investigation of adrenocortical insufficiency.
Administration :
TETRACOSACTIDE INJECTION B.P. is for I.M./I.V. administration.
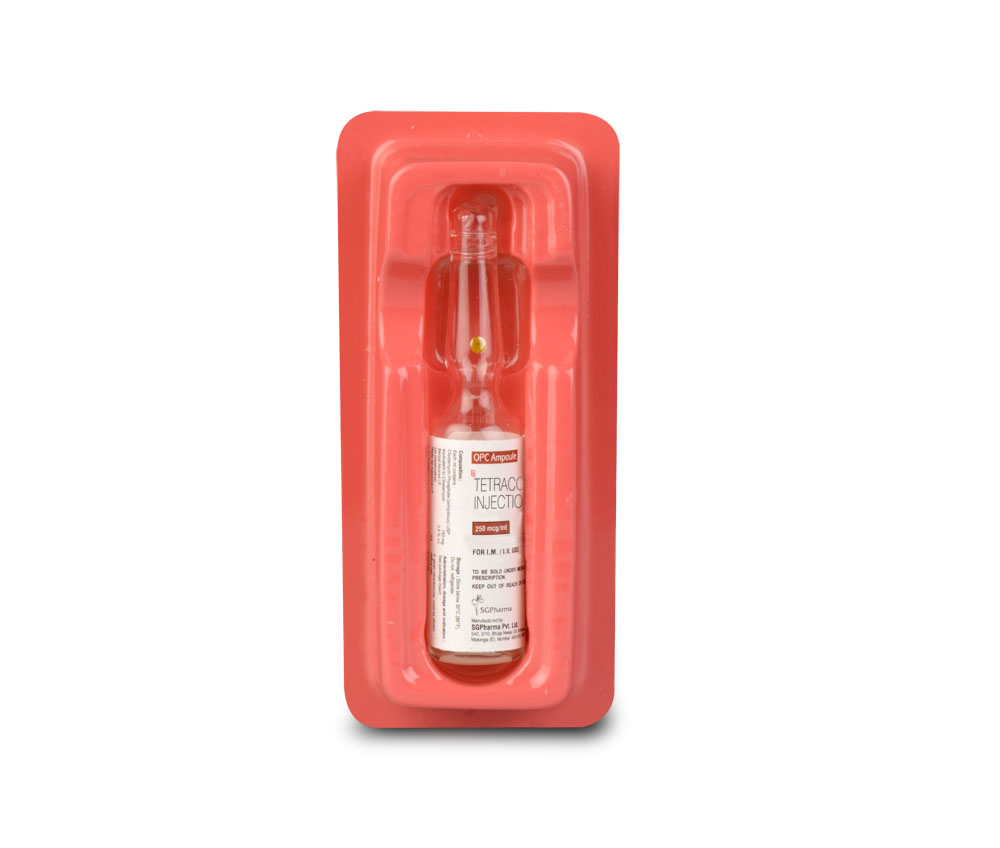
INSTRUCTIONS FOR USE OF AMPOULE :
The ampoule used in this product is equipped with O.P.C (One Point Cut) opening system. No ampoule file is needed to open the ampoule. The neck of the ampoule is prescored at the point of constriction. A coloured dot on the ampoule head helps to orientate the ampoule. Take the ampoule and face the coloured dot. Let the solution at the head of the ampoule to flow down by shaking or a gentle stroke. The ampoule opens easily by placing the thumb on the coloured dot and gently pressing downwards as shown.

Dosage :
Adults : This preparation of TETRACOSACTIDE INJECTION B.P. is intended for administration for diagnostic purposes only as a single intramuscular or intravenous dose; it is not to be used for repeated therapeutic administration. The 30-minute TETRACOSACTIDE INJECTION B.P. diagnostic test : This test is based on measurement of the plasma cortisol concentration immediately before and exactly 30 minutes after an intramuscular or intravenous injection of 250 micrograms (1 ml) TETRACOSACTIDE INJECTION B.P. Adrenocortical function can be regarded as normal if the post-injection rise in plasma cortisol concentration amounts to at least 200 nmol/litre (70 micrograms/litre). Where the 30-minute test has yielded inconclusive results, or where it is desired to determine the functional reserve of the adrenal cortex, a 5-hour test can be performed with TETRACOSACTIDE INJECTION B.P. Furthermore, a 3-day test with TETRACOSACTIDE INJECTION B.P. may be used to differentiate between primary and secondary adrenocortical insufficiency.
Children : An intravenous dose of 250 micrograms/1.73m² body surface area has been suggested. Thus for children aged 5 to 7 years, approximately half the adult dose will be adequate. For more accurate dosing of other ages, standard body surface area tables should be consulted.
Elderly : There is no evidence to suggest that dosage should be different in the elderly.
CONTRAINDICATIONS :
History of hypersensitivity to Adrenocorticotropic hormone (ACTH), TETRACOSACTIDE INJECTION B.P. TETRACOSACTIDE INJECTION B.P. is contra-indicated in patients with allergic disorders (e.g. asthma), acute psychosis, infectious diseases, peptic ulcer, refractory heart failure, Cushing’s syndrome, and primary adrenocortical insufficiency. TETRACOSACTIDE INJECTION B.P. should not be used during pregnancy or lactation unless there are compelling reasons to do so.
WARNINGS AND PRECAUTIONS :
Before using TETRACOSACTIDE INJECTION B.P., the doctor should make every effort to find out whether the patient is suffering from, or has a history of, allergic disorders. In particular, he should enquire whether the patient has previously experienced adverse reactions to ACTH, TETRACOSACTIDE INJECTION B.P. or other drugs. TETRACOSACTIDE INJECTION B.P. should only be administered under the supervision of appropriate senior hospital medical staff (e.g. consultants). If local or systemic hypersensitivity reactions occur after the injection (for example, marked redness and pain at the injection site, urticaria, pruritus, flushing, faintness or dyspnoea), TETRACOSACTIDE INJECTION B.P. or other ACTH preparations should be avoided in the future. Hypersensitivity reactions tend to occur within 30 minutes of an injection. The patient should therefore be kept under observation during this time.
Preparation should be made in advance to combat any anaphylactic reaction that may occur after an injection of TETRACOSACTIDE INJECTION B.P.. In the event of a serious anaphylactic reaction occurring, the following measures must be taken immediately : administer adrenaline (0.4 to 1 ml of a 0.1 % solution intramuscularly or 0.1 to 0.2 ml of a 0.1 % solution in 10 ml physiological saline slowly intravenously) as well as a large intravenous dose of a corticosteroid (for example 100 mg to 500 mg hydrocortisone, three or four times in 24 hours), repeating the dose if necessary. The hydrocortisone product information prepared by the manufacturer should also be consulted.
TETRACOSACTIDE INJECTION B.P. should not be used in the presence of active infectious or systemic diseases, when the use of live vaccine is contemplated or in the presence of a reduced immune response, unless adequate disease specific therapy is being given.
Use with care in patients with hypertension and thromboembolic tendencies. The increased production of adrenal steroids may result in corticosteroid type effects :
• Psychological disturbances may be triggered or aggravated.
• Latent infections (e.g. amoebiasis, tuberculosis) may become activated.
• Ocular effects may be produced (e.g. glaucoma, cataracts).
• Dosage adjustments may be necessary in patients being treated for diabetes or hypertension.
Pregnancy : Category C
There is limited amount of data in the use of tetracosactide in pregnant women. Animal studies are insufficient with respect to reproductive toxicity. Usage in pregnancy is contraindicated. Therefore the tetracosactide should not be utilised during pregnancy and lactation unless there are compelling reasons for doing so.
Nursing mothers :
It is not known whether tetracosactide enters breast milk or not. Usage in lactation is contraindicated.
Paediatric use :
Appropriate studies on the relationship of age to the effects of tetracosactide have not been performed in the paediatric population. However, no paediatrics-specific problems have been documented to date.
EFFECTS ON ABILITY TO DRIVE AND USE MACHINES :
Patients should be warned of the potential hazards of driving or operating machinery if they experience side effects such as dizziness.
INTERACTIONS :
Observed interactions resulting in concomitant use not being recommended Severe jaundice has been observed for concurrent use of tetracosactide and valproate in paediatric population. Their concurrent use should be avoided.
Observed interactions to be considered
Concurrent use of tetracosactide and other anticonvulsants (e.g. phenytoin, clonazepam, nitrazepam, phenobarbital, primidone) may increase the risk of liver damage, thus, TETRACOSACTIDE INJECTION B.P. should be used with caution at minimum possible doses and for minimum duration for concurrent treatment. Endogenous and synthetic estrogens can cause an increase in total cortisol levels and therefore, it is considered appropriate to use alternative methods (e.g., salivary cortisol, free cortisol index, plasma free cortisol) for interpretation of the results of the HPA axis examination.
Anticipated interactions to be considered
Since tetracosactide increases the adrenocortical production of glucocorticoids and mineralocorticoids, drug interactions of the type seen with these corticosteroids may occur. Patients already receiving medication for diabetes mellitus or for moderate to severe hypertension must have their dosage adjusted if treatment with tetracosactide is started.
SIDE EFFECTS :
The following side effects have been selected on the basis of their potential clinical significance (possible signs and symptoms in parentheses where appropriate) -not necessarily inclusive :
Those indicating need for medical attention
Incidence rare
Anaphylaxis, generalized (dizziness; hives; irritability; itching of skin; light headedness; seizures; skin rash; slow heartbeat; trouble in breathing; wheezing) Those indicating need for medical attention only if they continue or are bothersome Incidence less frequent or rare
Allergic reaction, mild (mild fever; nausea; vomiting) ; redness or pain at injection site
OVERDOSAGE :
If signs of water retention (increase in body weight) or excessive adrenocortical activity (Cushing’s syndrome) appear, TETRACOSACTIDE INJECTION B.P. should either be withdrawn for a while or given in lower doses.
TREATMENT OF OVERDOSAGE :
There is no known antidote. Symptomatic treatment is indicated.
PHARMACEUTICAL PRECAUTION :
Parenteral drug products should be inspected visually for particulate matter and discolouration prior to administration, whenever solution and container permit.
STORAGE :
Store in a refrigerator between 2°C to 8°C (36°F to 46°F), protected from light.
Do not freeze.
SHELF LIFE :
24 months from the date of manufacture.
PRESENTATION :
TETRACOSACTIDE INJECTION B.P. is supplied as 250 mcg of Tetracosactide (as acetate) B.P. in 1 ml Solution.
Such one ampoule of 1 ml is packed in a Box.
Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.

 Cardiovascular
Cardiovascular