200 mg/5 ml
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
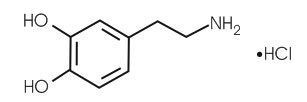
DOPASOL (Dopamine Hydrochloride) is a naturally occurring biochemical catecholamine precursor of noradrenaline and adrenaline. Chemically Dopamine HCl is 4-(2-Aminoethyl)pyrocatechol hydrochloride. The molecular formula is C8H11NO2.HCl and its molecular weight is 189.64.
STRUCTURAL FORMULA :
Its structural formula is :
DOPASOL is a clear colourless or pale yellow solution, filled in 5 ml flint ampoules. It contains no preservatives.
COMPOSITION :
Each ml contains :
Dopamine HCl USP 40 mg
Water for Injections I.P. q.s.
Contains no preservatives.
ACTIONS :
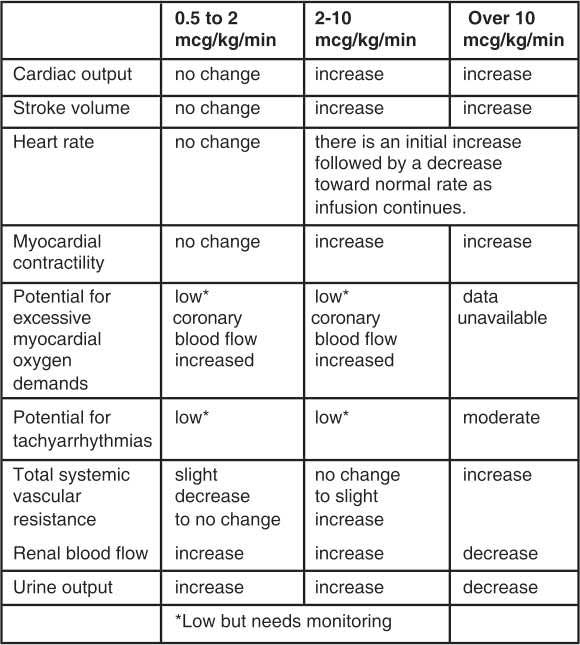
Dopamine hydrochloride can stimulate alpha, beta and Dopamine receptors. At infusion rates of 0.5 to 2 micrograms/kg/min, dopamine receptors are selectively activated and blood pressure either does not change or decreases slightly. The most important effects are renal and mesenteric vasodilatation. Renal plasma flow, glomerular filtration rate and sodium excretion usually increase. At infusion rates of 2 to 10 micrograms/kg/min, beta1-receptors are activated and cardiac output and systolic blood pressure increase. The total peripheral resistance is relatively unchanged because of peripheral vasoconstriction (alpha effect) and muscle vasodilatation (beta effect). At infusion rates above 10 micrograms/kg/min, alpha-receptors are activated, causing vasoconstriction, and both systolic and diastolic pressures increase. Dopamine does not cross the blood-brain barrier and so does not activate Dopamine receptors in the brain.
Cardiovascular effects of Dopamine at various infusion rates
PHARMACOKINETICS :
Absorption :
The steady state blood levels following intravenous infusion have not been determined in any species, nor has the time for these to be achieved.
Distribution :
Dopamine is widely distributed in the body.
Protein binding :
No information is available for humans or animals (however, Dopamine is rapidly metabolised and excreted).
Metabolism :
Dopamine is metabolised in the liver, kidneys and plasma and the metabolites are excreted by the kidneys. The major routes of metabolism are deamination by monoamine oxidase and formation of methylated and reduced derivatives by catechol-o-methyl transferase. On infusion of 14C labelled Dopamine into humans, it was found that approximately 75 % of the infused Dopamine was rapidly converted into metabolites of Dopamine and 25 % was synthesised into noradrenaline and its metabolic products. Only a trace of unlabelled adrenaline was detected. The principal metabolite of Dopamine was 3-methoxy-4-hydroxy phenylethanol (18.6 % of an infused dose) and the principal metabolites of noradrenaline were normetanephrine and 3-4-dihydroxy-mandelic acid.
For the correction of haemodynamic imbalance present in : Acute hypotension or shock associated with myocardial infarction, endotoxic septicaemia, trauma and renal failure. As an adjunct after open heart surgery, where there is persistent hypotension after correction of hypovolaemia. In chronic cardiac decompensation as in congestive failure.
Administration :
NOT FOR DIRECT INTRAVENOUS INJECTION.
DOPASOL is a potent drug. It must be diluted before administration. DOPASOL is incompatible with alkaline solutions and it is inactivated by 5 % Sodium bicarbonate. It is also incompatible with alkaline drugs such as Frusemide and Thiopentone sodium. Incompatibility of Dopamine with Insulin, Ampicillin and Amphotericin also has been reported. Being a powerful vasoconstrictor, it cannot be administered by IM or SC route.
INSTRUCTIONS FOR USE OF AMPOULE :
The ampoule used in this product is equipped with enhanced snap-off opening system. No ampoule file is needed to open the ampoule. The neck of the ampoule is prescored at the point of constriction. Take the ampoule, let the solution at the head of ampoule to flow down by shaking or a gentle stroke. The ampoule opens easily by placing the thumb on the head and gently pressing downwards as shown.

In appropriate cases, restoration of blood volume with plasma, whole blood, or a suitable plasma expander, should be instituted prior to administration : central venous pressure should be 10 to 15 cm H2O, or pulmonary wedge pressure 14 to 18 mm Hg.
Mode of administration :
The rate of administration should be controlled in order to prevent inadvertent bolus administration : constant evaluation of therapy should be undertaken (i.e. blood volume, ECG, arterial blood pressure, urine output, augmentation of myocardial contractility and distribution of peripheral perfusion. Measurement of central venous pressure and pulmonary wedge pressure and cardiac output are also helpful). Dopamine should be administered into a large vein (preferably of the antecubital fossa) to reduce the risk of extravasation into surrounding tissue which may cause necrosis.
Antidote for peripheral ischaemia following extravasation :
To prevent sloughing and necrosis in ischaemic areas, the area should be infiltrated as soon as possible with 10 to 15 mL of sodium chloride intravenous infusion 0.9 % containing from 5 to 10 milligrams of phentolamine, an adrenergic blocking agent. A syringe with a fine hypodermic needle should be used, and the solution liberally infiltrated throughout the ischaemic area. Sympathetic blockade with phentolamine causes immediate and conspicuous local hyperaemic changes if the area is infiltrated within 12 hours. Therefore, phentolamine should be given as soon as possible after the extravasation is noted.
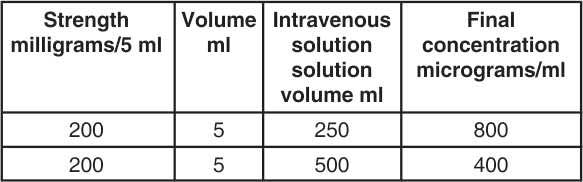
Suggested dilution :
Aseptically transfer Dopamine HCl Injection into the intravenous solution as per the table below :
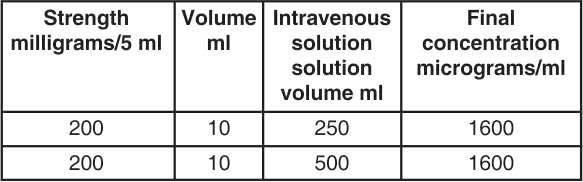
In patients in whom greater fluid load is undesirable, an alternative regimen is suggested :
Rate of administration :
Dopamine, after dilution, is administered intravenously through a suitable intravenous catheter or needle. An intravenous drip chamber or other suitable metering device is essential for controlling the rate of flow in drops per minute. Each patient must be individually titrated to the desired haemodynamic and/or renal response with Dopamine. In titrating to the desired increase in systolic blood pressure, the optimum dosage rate for renal response may be exceeded, thus, necessitating a reduction in rate after the haemodynamic condition is stabilised. Administration at rates greater than 50 micrograms/kg/minute has safely been used in advanced circulatory decompensation states. If unnecessary fluid expansion is of concern, adjustment of drug concentration may be preferred over increasing the flow rate of a less concentrated dilution.
Adult dosage :
When appropriate, increase blood volume with a suitable plasma expander, whole blood or plasma until central venous pressure is 10 to 15 cm H2O or pulmonary wedge pressure is 14 to 18 mm Hg. Begin administration of diluted solution at doses of 2 to 5 micrograms/kg/min in patients who are likely to respond to modest increments of heart force and renal perfusion. In more seriously ill patients, begin administration of diluted solution at doses of 5 micrograms/kg/min and increase gradually using 5 to 10 micrograms/kg/min increments up to 20 to 50 micrograms/kg/min as needed. In patients who do not respond to these doses with adequate arterial pressures or urine flow, additional increments may be employed in an effort to produce an appropriate arterial pressure and central perfusion. If doses of Dopamine in excess of 50 micrograms/kg/min are required, it is suggested that urine output be checked frequently. Should urine flow begin to decrease in the absence of hypotension, reduction of dosage should be considered. Once optimal haemodynamic effects have been achieved, the lowest dose that maintains these effects should be used. Multiclinic trials have shown that more than 50 % of the patients were satisfactorily maintained on doses of Dopamine less than 20 micrograms/kg/min.
Paediatric :
It is not recommended for use in children as safety and efficacy in this age group has not been established.
Geriatric :
No variation in dosage is suggested for geriatric patients. However, close monitoring is required for blood pressure, urine flow, and peripheral tissue perfusion.
With impaired hepatic function :
Dopamine is metabolised in the tissues and blood by MAO and COMT. Since the effect of impaired liver function is not known, close monitoring is advisable.
With impaired renal function :
Dopamine and its metabolites are almost completely excreted in the urine. Since the effect of impaired renal function is not known, close monitoring of such patients is advisable.
Patients who are taking monoamine oxidase inhibitors or who have taken them within the last two to three weeks require a substantially reduced starting dose, i.e. about 1/10th the usual dose. DOPASOL should not be added to alkaline diluents. Hypovolaemia should be fully corrected prior to treatment with Dopamine with a suitable plasma expander or whole blood or plasma until the central venous pressure is 10 to 15 cm H2O or the pulmonary wedge pressure is 14 to 18 mm Hg. Excessive dosage may be indicated by a disproportionate rise in diastolic pressure (i.e. a marked decrease in pulse pressure). The infusion rate should be decreased or ceased. Those patients with pre-existing peripheral vascular disease, such as that due to atherosclerosis, arterial embolism, Buergers disease, Raynauds disease, diabetic endarteritis or cold injury (e.g. Frostbite), may be more susceptible to peripheral ischaemia and subsequent gangrene and should be observed carefully for any changes in colour or temperature of the skin in the extremities. If ischaemia occurs and is thought to be due to vasoconstriction, the benefits of the Dopamine infusion should be weighed against the risks of possible necrosis. Ischaemia may be reversed by either decreasing the rate or discontinuing the infusion. Intravenous administration of phentolamine 5 to 10 milligrams may also reverse the ischaemia.
Usage in Pregnancy : Pregnancy category B3 Drugs which have been taken by only a limited number of pregnant women and women of childbearing age, without an increase in the frequency of malformation or other direct or indirect harmful effects on the human foetus having been observed. Studies in animals have shown evidence of increased foetal damage, the significance of which is considered uncertain in humans.
Labour and Delivery :
It is not known whether Dopamine crosses the placental barrier. In one animal study, the administration of Dopamine to pregnant rats resulted in a decreased survival rate of the newborn and cataract formation in the survivors. The benefits of using this product should be weighed against the possible risks to the foetus.
Nursing Mothers :
It is not known if Dopamine is excreted in breast milk, nor is the effect on the infant known. Dopamine is inactive when ingested orally, nonetheless it is not recommended for breast-feeding mothers unless the expected benefits outweigh any potential risks.
Other :
The genotoxic potential of Dopamine has not been evaluated. Long term studies in animals have not been performed to evaluate the carcinogenic potential of Dopamine. Studies in animals have not been performed to assess the effects of Dopamine on fertility.
DRUG INTERACTION :
Cyclopropane and halogenated anaesthetics sensitise the myocardium to the effects of Dopamine. Dopamine should therefore be used with extreme caution with these drugs due to the potential for developing ventricular arrhythmias or hypertension. Monoamine oxidase (MAO) inhibitors potentiate the effect of Dopamine and prolong its duration of action. Patients being treated, or who have been treated within the previous two to three weeks, with MAO inhibitors will, therefore, require a substantially reduced dosage of Dopamine. (The starting dose should be reduced to 1/10th of the usual dose or less). Alpha and beta adrenergic receptor blocking drugs will interfere with the alpha and beta adrenergic responses induced by Dopamine. The use of other pressor amines may be associated with complex interactions. Hypotension may be observed with concurrent use of vasodilators such as Glyceryl Trinitrate, Nitroprusside and Calcium channel blockers. In animal studies, large doses of butyrophenones blocked the dopaminergic mediated renal vasodilation. Whether this occurs in man is not known. Tricyclic antidepressants may potentiate the cardiovascular effects of Dopamine, possibly resulting in arrhythmias, tachycardia or severe hypertension or hyperpyrexia. Concurrent use of digitalis glycosides with Dopamine may increase the risk of cardiac arrhythmias. Caution and close ECG monitoring are very important if concurrent use is necessary.
Interference with laboratory tests :
Dopamine or its metabolites may interfere with urine tests for amino acids or catecholamines and also with tests for detecting uric acid or urobilinogen.
SIDE EFFECTS :
Common reactions :
Adverse reactions have been observed in 19 % of patients during clinical evaluation; however, only half of these were attributed to Dopamine. Treatment was terminated in 5 % of all patients due to adverse reactions.
Cardiovascular : Ectopic beats, tachycardia, anginal pain, palpitation, hypotension, vasoconstriction.
Gastrointestinal : Nausea, vomiting.
Nervous system : Headache.
Respiratory : Dyspnoea.
Less common reactions :
Biochemical abnormalities : Azotaemia.
Cardiovascular : Aberrant ventricular conduction, bradycardia, widened QRS complex, hypertension. Gangrene of the feet has occurred in a few patients with pre-existing vascular disease. A few cases of peripheral cyanosis have been reported in patients receiving Dopamine.
Serious or life threatening reactions :
Gangrene of feet has occurred following doses of 10 to 14 micrograms/kg/min and higher and in a few patients with pre-existing vascular disease. Fatal ventricular arrhythmias have been reported on rare occasions. Extravasation of Dopamine may cause necrosis and sloughing of surrounding tissue.
OVERDOSAGES :
Signs and Symptoms :
Excessive elevation of blood pressure could be expected from accidental overdose.
TREATMENT OF OVERDOSAGE :
In case of accidental overdose the rate of administration should be reduced or the infusion discontinued temporarily until the patients condition stabilises. Since the duration of action of Dopamine is quite short, no additional measures are usually necessary. If these measures fail to stabilise the patients condition in a relatively short time, use of the short acting alpha adrenergic blocking agent, Phentolamine, should be considered.
PHARMACEUTICAL PRECAUTIONS :
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Solutions which are darker than slightly yellow should not be used.
STORAGE :
Store below 30°C (86°F), protected from light.
Do not refrigerate.
SHELF LIFE :
24 months from the date of manufacture.
PRESENTATION :
DOPASOL is supplied as 200 mg Dopamine in 5 ml aqueous solution.
Such 5 ampoules of 5 ml are packed in a Box.
Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.

 Cardiovascular
Cardiovascular