50 mcg/ml, 500 mcg/ml
10 mg/20 ml, 20 mg/20 ml
40 mg/20 ml
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
BACLOFEN INJECTION (Baclofen) is a muscle relaxant and antispastic. Chemically, Baclofen is β-(Aminomethyl)-p-chlorohydrocinnamic acid. The molecular formula is C10H12ClNO2 and molecular weight is 213.66.
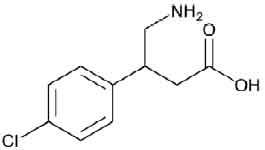
STRUCTURAL FORMULA :
Its structural formula is :
BACLOFEN INJECTION is a sterile, clear, colourless solution filled in amber ampoule / vial of suitable size.
COMPOSITION :
Each ml contains :
Baclofen USP 50 mcg
Sodium Chloride USP 9 mg
Water for Injection USP q.s.
Each ml contains :
Baclofen USP 500 mcg
Sodium Chloride USP 9 mg
Water for Injection USP q.s.
Each ml contains :
Baclofen USP 1000 mcg
Sodium Chloride USP 9 mg
Water for Injection USP q.s.
Each ml contains :
Baclofen USP 2000 mcg
Sodium Chloride USP 9 mg
Water for Injection USP q.s.
ACTIONS :
Baclofen, an analogue of gamma-aminobutyric acid, is a centrally acting skeletal muscle relaxant. It interferes with the release of excitatory neurotransmitters and inhibits monosynaptic and polysynaptic transmission at the spinal cord level. It may also act at supraspinal sites producing CNS depression. Baclofen is one of the drugs commonly used for the symptomatic relief of severe chronic spasticity associated with a variety of conditions.
PHARMACOKINETICS :
Because of the slow CSF circulation and the baclofen concentration gradient from the lumbar to the cisternal CSF the pharmacokinetic parameters observed in this fluid and as described below should be interpreted considering a high inter-and intra-patients variability.
Absorption :
Direct infusion into the spinal subarachnoid space by-passes absorption processes and allows exposure to the receptor sites in the dorsal horn of the spinal cord.
Onset of Action :
Intrathecal bolus : The onset of action is generally half an hour to one hour after administration of a single intrathecal dose of baclofen. Peak spasmolytic effect is seen at approximately 4 hours after dosing, the effect lasting 4 to 8 hours. Onset, peak response and duration of action may vary with individual patients depending on the severity of symptoms and the dose,method and speed of drug administration.
Continuous infusion :
Baclofen’s antispasmodic action is first seen 6 to 8 hours after initiation of continuous infusion. Maximum efficacy is observed within 24 to 48 hours.
Distribution :
After a single intrathecal bolus injection/rapid infusion, the distribution volume calculated from the concentration in the CSF ranges from 22 to 157 ml. The mean of about 75 ml corresponds approximately to the human CSF volume, and indicates that it is this in which the baclofen is mainly distributed. With continuous intrathecal infusion of daily doses of between 50 to 1200 microgram, steady-state concentrations of baclofen in the CSF of the lumbar region of 130 to 1240 nanogram/ml are reached within 1 to 2 days. In the steady state with continuous intrathecal infusion of daily doses between 95 to 190 microgram, a mean baclofen concentration gradient from lumbar to cisternal of 4 : 1 (range 8.7 : 1-1.8 : 1) is found. This is independent of the body position of the patient. All three strengths of baclofen solution (density: 1.003 ± 0.001 g/cm3 at 23°C) are practically isobaric to human CSF (density : 1.006-1.008 g/cm3). The baclofen plasma concentrations under intrathecal infusion of clinically used doses of baclofen are below 5 nanogram/ml (≤ 10 nanogram/ml in children) and are thus below the analytical quantitation limits. From this it can be concluded that baclofen crosses the blood-brain barrier only slowly and becomes systemically available only in very small quantities.
Elimination :
The pharmacokinetics of Cerebrospinal Fluid (CSF) clearance of baclofen, calculated from intrathecal bolus or continuous infusion studies, approximate CSF turnover, suggesting elimination is by bulk-flow removal of CSF. After both single bolus injection and chronic lumbar subarachnoid infusion using an implantable pump system, the mean CSF clearance is about 30 ml/h. The elimination half-life in the CSF after single intrathecal bolus injection/short-term infusion of 50 to 136 micrograms baclofen ranges from 1 to 5 hours. The elimination half-life of baclofen in the CSF at steady-state has not been de termined.
Special populations
Elderly Patients
No pharmacokinetic data is available in elderly patients after administration of BACLOFEN INJECTION Intrathecal. When a single dose of the oral formulation is administered, data suggest that elderly patients have a slower elimination but a similar systemic exposure to Baclofen compared to young adults. However, the extrapolation of these results to multi-dose treatment suggests no significant pharmacokinetics difference between young adults and elderly patients.
Paediatrics
In paediatric patients, respective plasma concentrations are at or below 10 ng/ml.
Hepatic impairment
No pharmacokinetic data is available in patients with hepatic impairment after administration of BACLOFEN INJECTION Intrathecal. However, as liver does not play a significant role in the disposition of baclofen it is unlikely that its pharmacokinetics would be altered to a clinically significant level in patient with hepatic impairment.
Renal impairment
No pharmacokinetic data is available in patients with renal impairment after administration of BACLOFEN INJECTION Intrathecal. Since baclofen is majorly eliminated unchanged through the kidneys, accumulation of unchanged drug in patients with renal impairment cannot be excluded.
INDICATIONS :
BACLOFEN INJECTION used for the treatment of severe chronic spasticity associated with multiple sclerosis, with injuries to the spinal cord or of cerebral origin that cannot be treated successfully with a standard treatment.
Paediatric population
BACLOFEN INJECTION is indicated in patients aged 4 to < 18 years with severe chronic spasticity of cerebral origin or of spinal origin (associated with injury, multiple sclerosis, or other spinal cord diseases) who are unresponsive to orally administered antispastics (including oral baclofen) and/or who experience unacceptable side effects at effective oral doses.
Administration :
BACLOFEN INJECTION is for Intrathecal administration. Intrathecal administration of baclofen through an implanted delivery system should only be undertaken by physicians with the necessary knowledge and experience. Specific instructions for implanting, programming and/or refilling the implantable pump are given by the pump manufacturers, and must be strictly adhered to.
INSTRUCTIONS FOR USE OF AMPOULE :
The ampoule used in this product is equipped with O.P.C (One Point Cut) opening system. No ampoule file is needed to open the ampoule. The neck of the ampoule is prescored at the point of constriction. A coloured dot on the ampoule head helps to orientate the ampoule. Take the ampoule and face the coloured dot. Let the solution at the head of the ampoule to flow down by shaking or a gentle stroke. The ampoule opens easily by placing the thumb on the coloured dot and gently pressing downwards as shown.

Dosage :
BACLOFEN INJECTION is intended for administration in single bolus test doses (via spinal catheter or lumbar puncture) and, for chronic use, in implantable pumps suitable for continuous administration of baclofen solution into the intrathecal space. For patients with spasticity due to head injury, it is recommended not to proceed to long-term BACLOFEN INJECTION therapy until the symptoms of spasticity are stable (i.e. at least one year after the injury). Establishment of the optimum dose schedule requires that each patient undergoes an initial screening phase with test doses by intrathecal bolus, followed by a very careful individual dose titration prior to maintenance therapy. This is due to the great variability in the effective individual therapeutic dose. Patients must be monitored closely in a fully equipped and staffed environment during the screening phase and dose-titration period immediately following implant. Resuscitative equipment should be available for immediate use in case of life-threatening or intolerable adverse reactions. Implantation of pumps should only be performed by experienced clinicians in properly equipped centres in order to minimise the risks in the perioperative phase .
Screening phase :
Prior to initiation of chronic infusion of intrathecal baclofen, patients must demonstrate a response to an intrathecal bolus of baclofen in a screening trial. A bolus test dose of Baclofen is usually administered via a lumbar puncture or an intrathecal catheter to elicit a response. In adults, the usual initial test dose is 25 micrograms or 50 micrograms and is stepped up by 25 microgram increments at least 24 hours apart, until a response lasting approximately 4 to 8 hours is observed; the dose should be given by barbotage over at least one minute. In children, the recommended initial test dose is 25 micrograms. For the test dose, low concentration ampoules of 0.05 mg/ml are available. The first dose should be performed with resuscitative equipment on stand-by. Patients should demonstrate a significant decrease in muscle tone and/or frequency and/or severity of spasms in order to be considered responders to treatment. There is great variability in sensitivity to intrathecal baclofen. Signs of severe overdose (coma) have been observed in an adult patient after a single test dose of 25 micrograms. Patients who do not respond to a 100 microgram test dose should not be given further increases of dose or be considered for continuous intrathecal infusion.
Dose titration phase :
After confirmation that the patient is responsive to intrathecal baclofen by means of bolus test doses, intrathecal infusion is established using a suitable delivery system. To determine the initial total daily dose of intrathecal baclofen following implant, the screening dose which gave a positive effect should be doubled and administered over a 24-hour period, unless the efficacy of the bolus dose was maintained for more than 12 hours. In this case the starting daily dose should be the screening dose delivered over a 24-hour period. No dose increases should be administered in the first 24 hours. Patients with spasticity of spinal origin : After the first 24 hours, the dosage should be adjusted slowly on a daily basis to achieve the desired clinical effect, with dosage increments limited to 10 – 30 % to avoid possible overdosing. Patients with spasticity of cerebral origin : After the first 24 hours, the dosage should be adjusted slowly on a daily basis to achieve the desired clinical effect, with dosage increments limited to 5 - 15 % to avoid possible overdosing. With programmable pumps, the dose should be increased only once every 24 h. For nonprogrammable pumps with a 76 cm catheter delivering 1 ml/day, intervals of 48 hours are suggested for evaluation of response. If the daily dose has been substantially increased and no clinical effect is achieved, check for proper pump function and catheter patency. There is limited experience with doses greater than 1000 micrograms/day.
Maintenance therapy :
The clinical goal is to maintain as normal a muscle tone as possible and to minimise the frequency and severity of spasms without inducing intolerable side effects, or to titrate the dose to the desired degree of muscle tone for optimal function when patients with cerebral spasticity are treated. The lowest dose producing an adequate response should be used. Most patients require gradual increases in dose over time to maintain optimum response during chronic therapy due to decreased responsiveness to therapy or to progress of the disease. The retention of some spasticity is desirable to avoid a sensation of “paralysis” on the part of the patient. In addition, a degree of muscle tone and occasional spasms may help support circulatory function and possibly prevent the formation of deep vein thrombosis. Patients with spasticity of spinal origin : The daily dose may be gradually increased in steps of 10 – 30 % to maintain adequate symptom control by adjusting the dosing rate of the pump and/or the concentration of baclofen in the reservoir. The daily dose may also be reduced by 10-20 % if patients experience side effects. Patients with spasticity of cerebral origin : The daily dose may be gradually increased by 5 - 20 %, but no more than 20 %, to maintain adequate symptom control by adjusting the dosing rate of the pump
and/or the concentration of baclofen in the reservoir. The daily dose may also be reduced by 10 – 20 % if patients experience side effects.
A sudden requirement for substantial dose escalation suggests a catheter complication (i.e.,catheter kink or dislodgement) or pump malfunction. Most patients require gradual increases in dose over time to maintain optimum response during chronic therapy due to decreased responsiveness to therapy or to progress of the disease. Maintenance dosage for long-term continuous infusion of intrathecal baclofen in patients with spasticity of spinal origin ranges from 10 micrograms/day to 1200 micrograms /day, most patients being adequately maintained on 300 - 800 micrograms/day. In patients with spasticity of cerebral origin, the maintenance dosage for long-term continuous infusion of intrathecal baclofen ranges from 22 micrograms/day to 1400 micrograms/day, with a mean daily dose of 276 micrograms/day at 12 months and 307 micrograms/day at 24 months. Paediatric patients under twelve years of age generally require lower dosages than do older patients; the dose ranges from 24 - 1199 micrograms/day, with a mean daily dose of 274 micrograms/day.
Development of tolerance :
During long-term treatment approximately 5 % of patients become refractory to increasing doses. After a few days cessation of baclofen the sensitivity to baclofen may be restored and treatment should be resumed at the initial continuous infusion dose. This must be performed in a hospital unit Caution should be exercised when switching from BACLOFEN INJECTION Intrathecal to morphine and vice versa.
Special populations
Renal impairment :
No studies have been performed in patients with renal impairment with BACLOFEN INJECTION therapy. Because baclofen is primarily excreted unchanged by the kidneys it should be given with special care and caution in patients with impaired renal function.
Hepatic impairment :
No studies have been performed in patients with hepatic impairment receiving BACLOFEN INJECTION therapy. No dosage adjustment is recommended as the liver does not play any significant role in the metabolism of baclofen after intrathecal administration of BACLOFEN INJECTION. Therefore, hepatic impairment is not expected to impact the drug systemic exposure.
Geriatrics :
Several patients over the age of 65 years have been treated with BACLOFEN INJECTION during the clinical trials without increased risks compared to younger patients. Problems specific to this age group are not expected as doses are individually titrated. Instructions for Use / Handling
Ampoule specifications :
BACLOFEN INJECTION is intended for intrathecal injection and continuous intrathecal infusion. Each ampoule is intended for single use only. Discard any unused portion. Do not freeze. Do not heat-sterilise.
BACLOFEN INJECTION contains no preservatives. BACLOFEN INJECTION containing 0.05 mg/ml are available for administering low-dose bolus injections during the screening phase. BACLOFEN INJECTION containing 10 mg/5 ml are available for use with infusion pumps. The concentration chosen for use depends upon the total daily dose required as well as the delivery rate of the pump. Please consult the pump manufacturer’s manual for specific recommendations.
Dilution instructions :
For patients who require concentrations other than 0.05 mg/ml or 2 mg/ml, BACLOFEN INJECTION must be diluted, under aseptic conditions, with sterile preservative-free sodium chloride for injection.
CONTRAINDICATIONS :
BACLOFEN INJECTION is contraindicated
• Known hypersensitivity to Baclofen or to any of the excipients.
• The drug should not be administered by the intravenous, intramuscular, epidural or subcutaneous routes.
• Epilepsy refractory to therapy.
WARNINGS :
Psychotic disorders, confusional states, schizophrenia, depressive or manic disorders or Parkinson’s disease may be worsened by treatment with baclofen. Therefore, patients with these conditions should be kept under close observation and treatment should be
administered cautiously. Baclofen should be used with extreme care in patients already receiving antihypertensive therapy. Caution should be exercised with baclofen therapy in patients suffering from renal, hepatic or respiratory impairment or who have had a cerebrovascular accident. Patients with neurogenic disturbances affecting emptying of the bladder may show improvement in their condition whilst taking baclofen. However, patients with pre-existing sphincter hypertonia may suffer with acute urine retention during treatment with baclofen; as a result it should be used cautiously in these patients.
Appropriate laboratory tests should be carried out on patients with hepatic dysfunction or diabetes mellitus to make sure that no drug-induced changes to the underlying diseases have resulted with concomitant baclofen therapy as, rarely, elevated SGOT, alkaline phosphatase and glucose levels in serum have been recorded. Baclofen therapy should always be gradually discontinued, unless serious adverse effects have occurred, by reducing the dose over a period of 1-2 weeks. Anxiety, confusion, hallucinations, psychosis, mania, paranoia, convulsions, tachycardia, and, as a rebound phenomenon, temporary aggravation of spasticity, have all been reported on abrupt withdrawal. BACLOFEN INJECTION should be used cautiously in diabetic patients.
PRECAUTIONS :
Discontinuation of treatment :
No specific limit to the duration of treatment is foreseen. Except in emergencies due to overdosing or following development of serious adverse reactions, the treatment should always be discontinued by gradual reduction in the dosage. Baclofen must not be abruptly discontinued. In the event of abrupt discontinuation of intrathecal administration of baclofen, sequelae such as high fever, changes in mental state, increased spasticity as a rebound effect and muscle rigidity may occur regardless of the cause of the discontinuation, and in rare cases these may progress to seizures/status epilepticus, rhabdomyolysis, multiorgan failure and death.
Withdrawal symptoms :
In the event of abrupt discontinuation of intrathecal administration of baclofen, sequelae such as high fever, changes in mental state, increased spasticity as a rebound effect and muscle rigidity may occur regardless of the cause of the discontinuation, and in rare cases these may progress to seizures/status epilepticus, rhabdomyolysis, multiorgan failure and death. Discontinuation symptoms can possibly be confused with poisoning symptoms. They also require inpatient admission of the patient.
Therapy in the event of occurrence of withdrawal symptoms :
A rapid correct diagnosis and treatment in an emergency medical or intensive care unit are important to prevent the possibly life-threatening central nervous and systemic effects of withdrawal of intrathecal BACLOFEN INJECTION .
Safety considerations during use :
It is mandatory that the patient, the physicians responsible for the patient, and all those involved in the care of the patient receive adequate information about the risks of this mode of treatment. Physicians must be adequately trained in chronic intrathecal infusion therapy.
Everyone concerned with the treatment and care of the patient should be instructed on the signs and symptoms of overdose, procedures to be followed in the event of overdose, and proper home care of the pump and insertion site. For patients with spasticity due to head injury, it is recommended not to proceed to long-term BACLOFEN INJECTION therapy until the symptoms of spasticity are stable (i.e. at least one year after the injury).
Screening phase :
The pump system should not be implanted until the patient’s response to bolus intrathecal baclofen injection and/or dose titration is adequately evaluated and found to be clinically safe and effective. Because of the risks associated with the initial administration and
titration of intrathecal baclofen (CNS depression, cardiovascular collapse and/or respiratory failure), these steps must be conducted in a medically supervised and adequately equipped environment, following the instructions outlined in the Dosage and Administration section. The preliminary screening phase should be performed in a hospital and implantation of the pump system should be undertaken only in specialist units. Resuscitative equipment should be available for immediate use in case of life-threatening symptoms of severe overdose.
Careful monitoring of respiratory and cardiovascular functions is essential during administration of the initial test doses (screening phase), especially in patients with cardiopulmonary disease and respiratory muscle weakness as well as those being treated concomitantly with benzodiazepine-type preparations or opiates, who are at higher risk of respiratory depression. Patients should be infection-free prior to the screening trial with BACLOFEN INJECTION because the presence of a systemic infection may interfere with an assessment of the patient’s response to bolus BACLOFEN INJECTION Before use of the drug, myelography of the subarachnoid space should be performed in patients with postraumatic spasticity. If signs of arachnoiditis are detected, treatment should not be given.
Pump implantation and use :
Intrathecal administration of BACLOFEN INJECTION through an implanted delivery system should only be undertaken by physicians with the necessary knowledge and experience in properly equipped centres in order to minimise the risks in the perioperative period. Specific instructions for implanting, programming and/or refilling the implantable pump are given by the pump manufacturers, and must be strictly adhered to. Patients should be infection-free prior to pump implantation because the presence of infection may increase the risk of surgical complications. Moreover, a systemic infection may complicate attempts to adjust the dose. A local infection or catheter malplacement can also lead to drug delivery failure, which may result in sudden BACLOFEN INJECTION withdrawal and its related symptoms.
Reservoir refilling :
Reservoir refilling must be performed by qualified and fully trained personnel following the directions provided by the pump manufacturer. Strictly aseptic filling is required to avoid microbial contamination and serious infection. Refill intervals should be carefully calculated to prevent depletion of the reservoir, as this would result in the return of severe spasticity or potentially life-threatening symptoms of withdrawal . Extreme caution must be used when filling an implantable pump equipped with an injection port that allows direct access to the intrathecal catheter. Direct injection into the catheter through the access port may cause a life-threatening overdose.
Pregnancy : Category C
Not recommended in pregnancy as foetal malformations have been reported as having occurred in rats but not mice or rabbits. Where treatment is necessary, the benefits for the mother should be carefully considered against the possible risks to the child, particularly in the first trimester when baclofen should only be used if essential.
Nursing mothers :
Baclofen is not recommended whilst breast-feeding as it is known to be present in the milk.
EFFECTS ON ABILITY TO DRIVE AND USE MACHINES :
Patients taking BACLOFEN INJECTION should not take charge of vehicles, other means of transport, or machinery where loss of attention may lead to accidents.
INTERACTIONS AND INCOMPATIBILITIES :
There is little experience with the use of intrathecal BACLOFEN INJECTION in combination with systemic medications to predict specific drug-drug interactions, although it is suggested that the low baclofen systemic exposure observed after intrathecal administration could reduce the potential for pharmacokinetic interactions . Anticipated interactions resulting in concomitant use not being recommended Levodopa/DDC inhibitor : Concomitant use of oral BACLOFEN and levodopa/DDC inhibitor resulted in increased risk of adverse events like visual hallucinations, confusional state, headache and nausea. Worsening of the symptoms of Parkinsonism has also been reported. Thus, caution should be exercised when BACLOFEN INJECTION is administered to patients receiving levodopa/DDC inhibitor therapy. Observed interactions to be considered
Anaesthetic :
Concomitant use of BACLOFEN INJECTION and general anesthetics (e.g. fentanyl, propofol) may increase the risk of cardiac disturbances and seizures. Thus, caution should be exercised when anaesthetics are administered to patients receiving BACLOFEN NJECTION
Anticipated interactions to be considered .
Morphine :
The combined use of intramuscular morphine and BACLOFEN INJECTION was responsible for hypotension in one patient. The potential for this combination to cause dyspnoea or other CNS symptoms cannot be excluded. The co-administration of other intrathecal agents with intrathecal baclofen has not been tested and its safety is unknown. Alcohol and other compounds affecting the CNS The central nervous system depressant effects of alcohol and other compounds affecting the CNS (e.g. analgesics, neuroleptics, barbiturates, benzodiazepines, anxiolytics) may be additive to the effects of baclofen.
Tricyclic antidepressants
When using oral baclofen, concurrent treatment with tricyclic antidepressants may potentiate the effect of baclofen, resulting in pronounced muscular hypotonia. Caution is advised when using BACLOFEN INJECTION in this combination.
Antihypertensive
Since concomitant treatment with oral baclofen and antihypertensives is likely to further increase a possible fall in blood pressure, it may be necessary to monitor blood pressure and adjust the dosage of the antihypertensive medication accordingly.
Incompatibilities :
BACLOFEN INJECTION ampoules for intrathecal administration should not be mixed with other infusion or injection solutions. Glucose solutions are incompatible due to a chemical reaction with baclofen.
SIDE EFFECTS :
Side effects associated with Baclofen are often transient and dose-related. They may be minimised by increasing doses gradually or controlled by a reduction in dosage. The most common side-effects include drowsiness, nausea, dizziness, lassitude, lightheadedness, confusion, fatigue, muscular pain and weakness, and hypotension. Other side-effects include euphoria, hallucinations, depression, headache, tinnitus, convulsions, paraesthesias, slurred speech, dry mouth, taste alterations, vomiting, diarrhoea or constipation, ataxia, nystagmus, tremors, insomnia, visual disturbances, skin rashes, pruritus, increased sweating, urinary disturbances, respiratory or cardiovascular depression, blood sugar changes, alterations in liver function values, and a paradoxical increase in spasticity. Problems with erection and ejaculation have also been reported with intrathecal Baclofen ; these are usually reversible on withdrawal of therapy. Abrupt withdrawal of baclofen may result in a withdrawal syndrome
OVERDOSAGE :
Deaths due to overdose of Intrathecal BACLOFEN INJECTION have been reported. Special attention must be given to recognizing the signs and symptoms of overdosage at all times, especially during the initial “screening” and “dose-titration” phase of treatment but also during reintroduction of BACLOFEN INJECTION after a period of interruption of therapy. Signs of overdose may appear suddenly or insidiously. Serious overdose may occur for example by inadvertent delivery of catheter contents during catheter patency/position analysis. Errors in programming, excessively rapid dose increases and concomitant treatment with oral baclofen are other possible causes of overdosage. Possible pump malfunction should also be investigated. Symptoms of severe BACLOFEN INJECTION overdose (coma) were reported in a sensitive adult patient after receiving a 25 microgram intrathecal bolus dose.
Symptoms :
Excessive muscular hypotonia, drowsiness, lightheadedness, dizziness, somnolence, seizures, loss of consciousness, hypothermia, excessive salivation, nausea and vomiting. Respiratory depression, bradycardia, apnoea and coma result from serious overdosage.
TREATMENT OF OVERDOSAGE :
There is no specific antidote for treating overdoses of BACLOFEN INJECTION, but the following steps should generally be undertaken :
1. Residual baclofen solution should be removed from the pump as soon as possible.
2. Patients with respiratory depression should be intubated and ventilated, if necessary, until the drug is eliminated.
3. If lumbar puncture is not contraindicated, consideration should be given, in the early stage of the intoxication, to withdrawing 30 - 40 ml of CSF to reduce CSF Baclofen concentration.
4. Cardiovascular function should be supported.
5. In the event of convulsions, diazepam may be administered cautiously i.v.
PHARMACEUTICAL PRECAUTIONS :
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
STORAGE :
Store below 30°C (86°F), protected from light.
Do not refrigerate
SHELF LIFE :
24 months from date of manufacture.
PRESENTATION :
BACLOFEN INJECTION is supplied as below :

Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.

 Cardiovascular
Cardiovascular