200 mg, 250 mg,
400 mg, 800 mg
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
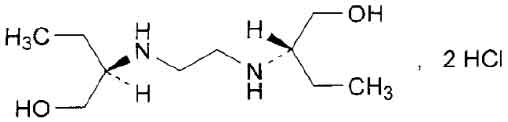
ETHAMBUTOL TABLETS B.P. (Ethambutol Hydrochloride) is indicated for the the treatment of pulmonary tuberculosis. It has also been used successfully in cases of primary tuberculosis and extrapulmonary forms of tuberculosis. Chemically, Ethambutol Hydrochloride is 2,2’-(ethylenediimino)bis[(2S)-butan-1-ol] dihydrochloride. The molecular formula is C10H26Cl2N2O2 and molecular weight is 277.2.
STRUCTURAL FORMULA :
Its structural formula is :
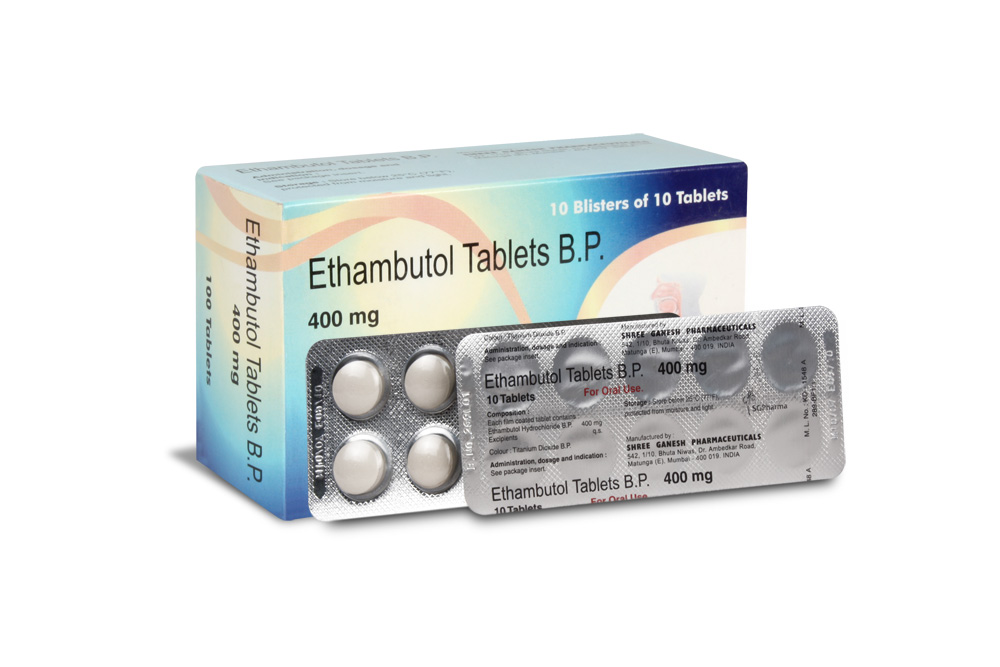
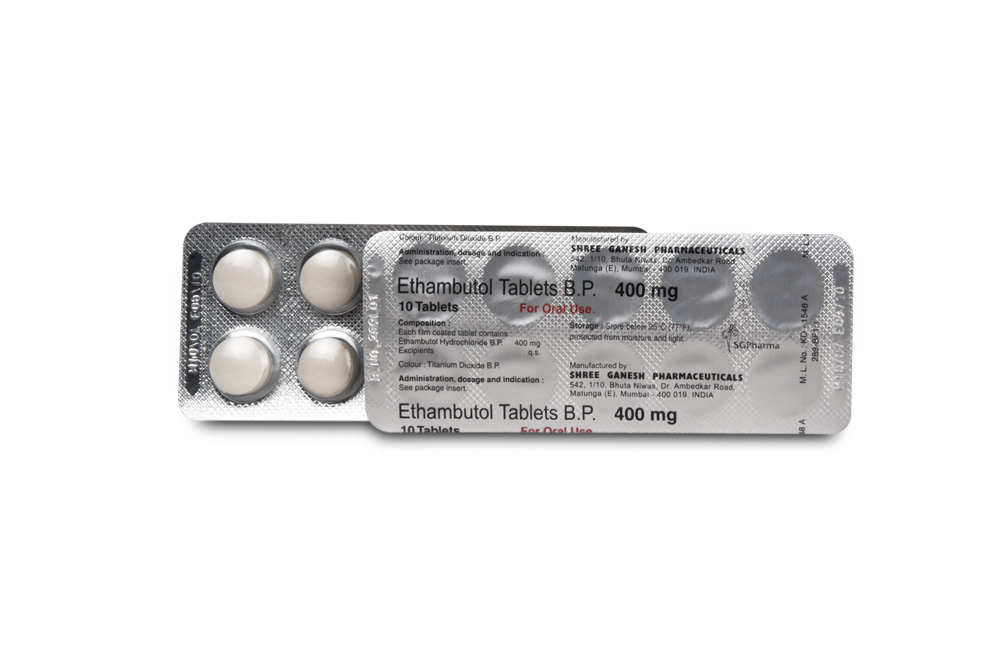
ETHAMBUTOL TABLETS B.P. 250 mg and 400 mg are white coloured, circular, biconvex film coated tablets.
COMPOSITION :
ETHAMBUTOL TABLETS B.P. 250 mg
Each film coated tablet contains :
Ethambutol Hydrochloride B.P. 250 mg
Excipients q.s.
Colour : Titanium Dioxide B.P.
ETHAMBUTOL TABLETS B.P. 400 mg
Each film coated tablet contains :
Ethambutol Hydrochloride B.P. 400 mg
Excipients q.s.
Colour : Titanium Dioxide B.P.
ACTIONS :
Ethambutol diffuses into actively growing Mycobacterium cells such as tubercle bacilli. Ethambutol appears to inhibit the synthesis of one or more metabolites, thus causing impairment of cell metabolism, arrest of multiplication and cell death. No cross resistance with other available antimycobacterial agents has been demonstrated. Ethambutol has been shown to be effective against strains of Mycobacterium tuberculosis but does not seem to be active against fungi, viruses or other bacteria. Mycobacterium tuberculosis strains previously unexposed to ethambutol have been uniformly sensitive to concentrations of 8 mcg/ml or less, depending on the nature of the culture media. When ethambutol has been used alone for the treatment of tuberculosis, tubercle bacilli from these patients have developed resistance to ethambutol as shown by in vitro susceptibility tests; the development of resistance has been unpredictable and appears to occur in a step-like manner. No cross resistance between ethambutol and other antituberculosis agents has been reported. Ethambutol has reduced the incidence of the emergence of mycobacterial resistance to isoniazid when both substances have been used concurrently.
PHARMACOKINETICS :
Approximately 80 % of an oral dose of ethambutol is absorbed from the gastro-intestinal tract, and the remainder is excreted unchanged in the faeces. Absorption is not significantly impaired by food. Peak serum levels of 2 to 5 mcg/ml occur in 2-4 hours following a single dose of 25 mg per kg body weight. When it is administered daily for longer periods of time at this dose, serum levels are similar. The serum levels of ethambutol fall to undetectable levels by 24 hours after the last dose, except in some patients with abnormal renal function.
Ethambutol is distributed in most tissues, including the lungs, kidneys and erythrocytes and is localised within pulmonary alveolar and axillary lymph node macrophages. One to two times as much ethambutol is present in the erythrocytes as in the plasma. Red blood cells may serve as a depot for which the substance slowly enters plasma. Ethambutol does not readily penetrate the blood-brain barrier, but it diffuses into the CSF when the meninges are inflamed.
During the 24 hour period following oral administration of ethambutol approximately 50 % of the initial dose is excreted unchanged in the urine, while an additional 8-15 % appears in the form of metabolites. The main path of metabolism occurs in the liver and appears to be an initial oxidation of the alcohol to an aldehydic intermediate followed by conversion to a dicarboxylic acid. Both metabolites are inactive and excreted in the urine. Both metabolites are devoid of antituberculosis activity are thought to be metabolised in the liver. Prolonged administration and elevated plasma concentrations do not lead to changes in metabolite production. Approximately 20 to 30 % of ethambutol is bound to plasma proteins over a plasma concentration range of 0.5 to 2.0 mcg/ml. With consecutive single daily doses of 25 mg/kg, no substance accumulation has been observed in patients with normal kidney function but marked accumulation has been demonstrated in patients with renal insufficiency.
INDICATIONS :
ETHAMBUTOL TABLETS B.P. is indicated for the treatment of pulmonary tuberculosis. It has also been used successfully in cases of primary tuberculosis and extrapulmonary forms of tuberculosis, including tuberculosis meningitis, genitourinary tuberculosis, miliary tuberculosis, tuberculosis of bones and joints, tuberculosis of the skin and tuberculosis eye diseases. ETHAMBUTOL TABLETS B.P. should not be used as the sole anti-tuberculosis agent, but used in conjunction with at least one other anti-tuberculosis treatment. For patients who have not received previous anti-tuberculosis therapy, the most frequently used regimens have included three of the following agents : Ethambutol, isoniazid, rifampicin and streptomycin for the first 2 to 4 months.
For example :
Ethambutol plus isoniazid plus rifampicin, or, Ethambutol plus isoniazid plus streptomycin,
Then continuing with a two agent regimen such as :
Ethambutol plus isoniazid, or,
Ethambutol plus rifampicin.
For patients who have received previous anti-tuberculosis therapy, mycobactrial resistance to other substances used in initial therapy is frequent. In such retreatment cases ethambutol should be combined with at least one of the second line agents not previously administered to the patient and to which bacterial susceptibility has been indicated by appropriate in vitro studies. Anti-tuberculosis agents used with ethambutol have included, cycloserine, ethionamide, pyrazinamide, viomycin and other substances. Isoniazide, aminosalicylic acid and streptomycin have also been used in multiple treatment regimens. Alternating treatment regimens have also been utilised.
Administration :
ETHAMBUTOL TABLETS B.P. is administered orally.
Dosage :
ETHAMBUTOL TABLETS B.P. should be used in conjunction with other antituberculosis agents such as isoniazid, rifampicin and pyrazinamide. ETHAMBUTOL TABLETS B.P. should only be administered once every 24 hours. Absorption of ethambutol is not significantly altered by administration with food.
Initial treatment :
In patients who have not received previous antituberculosis therapy, administer ETHAMBUTOL TABLETS B.P. 15 mg/kg of body weight, as a single oral dose once every 24 hours. Concurrently administer one other antituberculosis agent.
Retreatment :
In patients who have received previous antituberculosis therapy, administer ETHAMBUTOL TABLETS B.P. 25 mg/kg of body weight, as a single oral dose once every 24 hours. Concurrently administer at least one other antituberculosis agent. Suitable substances usually consist of those not previously used in the treatment of the patient. After 60 days of ETHAMBUTOL TABLETS B.P. administration, decrease the dose to 15 mg/kg of body weight, and administer as a single oral dose once every 24 hours. During the period when a patient is on a daily dose of 25 mg/kg, monthly eye examinations are advised.
Intermittent Therapy :
An alternative method of administration, in both initial and retreatment cases is to give the above mentioned daily doses of 15 or 25 mg/kg/day for two months or longer, depending on the type and extent of disease and the bacteriological and roentgenographic response (or until at least one negative sputum is obtained). Thereafter, ETHAMBUTOL TABLETS B.P. may be given in a dosage of 50 mg/kg twice weekly. When isoniazid is administered concomitantly, the usual dosage is 14 mg/kg twice weekly with 10 mg pyridoxine for each 100 mg of isoniazid. The usual daily dosage of isoniazid in adults is 300 mg or 5 mg/kg on the basis of body weight. In children the usual daily dosage is 5 to 20 mg/kg.
CONTRAINDICATIONS :
ETHAMBUTOL TABLETS B.P. is contraindicated in patients who are known to be hypersensitive to this substance. It is also contraindicated in patients with known optic neuritis unless clinical judgement determines that it may be used. No absolute contraindications to the administration of isoniazid have been reported.
WARNINGS :
Ethambutol should be given at a reduced dosage to patients with impaired kidney function. Ethambutol may have adverse effects on vision therefore physical examination should include ophthalmoscopy, finger perimetry and testing of colour discrimination. In patients with visual defects such as cataract, recurrent inflammatory conditions of the eye, optic neuritis and diabetic retinopathy, the evaluation of changes in visual acuity is more difficult and care should be taken to be sure the variations in vision are not due to the underlying disease conditions. Consideration should be given to the relationship between benefits expected and possible visual deterioration since evaluation of visual changes is difficult.
PRECAUTIONS :
Ethambutol should also be administered with caution to patients with gout. As with any potent substance, periodic assessment of organ functions, including the renal, hepatic and haematopoietic systems should be made during long term therapy. Toxicological studies in dogs given high doses for long periods showed evidence of myocardial damage and failure and depigmentation of the tapetum lucidum of the eye. The significance is not known. Degenerative changes of the central nervous system have also been noted in dogs receiving ethambutol over a prolonged period. In the rhesus monkey neurological signs appeared after treatment with high doses daily over a period of several months. These were correlated with specific serum levels of ethambutol hydrochloride and with definite neuroanatomical changes in the central nervous system. Focal interstitial carditis was also noted in monkeys receiving ethambutol hydrochloride in high doses for a prolonged period. Loss of fertility and testicular regression among male rats has been reported in the literature.
Use in Pregnancy : Category A.
The use of ethambutol during pregnancy has not been associated with foetal abnormalities however ethambutol cro Número de puntos disminuidos sses the placenta and may be teratogenic in animals. It is generally considered that the benefits of ethambutol in the treatment of tuberculosis outweigh any potential risks in pregnancy.
Use in Lactation :
Ethambutol is distributed into breast milk to produce concentrations similar to those in the plasma. Caution should be exercised when ethambutol is administered to nursing women.
INTERACTIONS AND INCOMPATIBILITIES :
Ethambutol does not appear to alter the pharmaceutical actions of other substances, and is commonly used with other antituberculosis agents to prevent the emergence of resistant strains of M. tuberculosis. Because of the effects of ethambutol on renal excretion of uric acid, the actions of uricosuric agents may be countered. Concomitant therapy with isoniazid and pyridoxine may contribute to the effects upon the excretion of uric acid.
SIDE EFFECTS :
Ethambutol may produce optic neuritis which decreases visual acuity and which appears to be related to dose and duration of treatment. Incidence with the recommended dosage averages about 2 to 3 % of patients. The effects are generally reversible when administration of ethambutol is discontinued promptly. In rare cases recovery may be delayed for one year or more and the effect may possibly be irreversible in these cases. Patients would be advised to report promptly to their physicians any change in visual acuity. The change in visual acuity may be unilateral or bilateral and therefore each eye must be tested separately and both eyes tested together. Before beginning ETHAMBUTOL TABLETS B.P. therapy visual acuity should be tested. Testing should then be done monthly once treatment of 15mg/kg/day or greater has begun. The following table could be useful in interpreting possible changes in visual acuity attributable to ETHAMBUTOL TABLETS B.P.
In general, changes in visual acuity less than those shown in the above table may be due to chance variation, limitations of the testing method or physiological variability. Also changes in visual acuity equalling or exceeding those shown in the table indicate the need for retesting and careful evaluation of the patient’s visual status. Recovery of visual acuity generally occurs over a period of weeks to months after treatment with ETHAMBUTOL TABLETS B.P. has been discontinued. Patients have then received ETHAMBUTOL TABLETS B.P. again without recurrence of loss of visual acuity.
Other adverse reactions reported include anaphylactoid reactions, dermatitis, pruritus, joint pain, anorexia, nausea, vomiting, gastrointestinal upset, abdominal pain, fever, malaise, headache, dizziness, mental confusion, disorientation and possible hallucinations. Numbness and tingling of the extremities due to peripheral neuritis have been reported infrequently. Rarely, Stevens-Johnson syndrome, toxic epidermal necrolysis. One case of reversible thrombocytopenia has been reported. Elevated serum uric acid levels and precipitation of acute gout have been reported. Pulmonary infiltrates and eosinophilia also have been reported. Transient impairment of liver function as indicated by abnormalities in liver function tests is not an unusual finding. Since ethambutol is used in conjunction with one or more other anituberculosis drugs, these changes may be related to concurrent therapy. Extensive tests of liver function led to the conclusion that ethambutol when given in recommended doses even for prolonged periods does not cause hepatic damage.
OVERDOSAGE :
Acute Overdosage Signs and Symptoms :
Vomiting, fever, gastrointestinal upset, abdominal pain, headache and dizziness, anorexia, nausea, malaise, mental confusion, disorientation and possible hallucinations. (NOTE : Anaphylactoid reactions may occur with usual therapeutic dosage).
TREATMENT OF OVERDOSAGE :
There is no specific antidote for ethambutol overdosage but the usual methods of gastric lavage and/or supportive therapy should be instituted. Blood concentrations may be reduced by haemodialysis or peritoneal dialysis. Anaphylactoid reactions may necessitate emergency treatment.
STORAGE :
Store below 300C(860F), protected from moisture and light.
SHELF LIFE :
24 months from the date of manufacture.
PRESENTATION :
ETHAMBUTOL TABLETS B.P. contains Ethambutol HCl B.P. 250 mg/400 mg
10 blisters of10 tablets per Box.
Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.

 Cardiovascular
Cardiovascular