0.25 mg
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
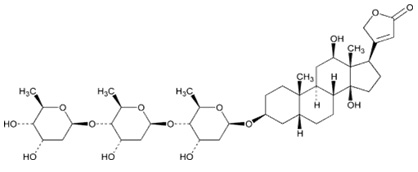
DIGOXIN TABLETS USP (Digoxin) is a cardiotonic glycoside obtained from the leaves of Digitalis lanata Ehrhart (Family- Scrophulariaceae). Chemically, digoxin is 3β-[(O-2,6-Dideoxy- β-d-ribo-hexopyranosyl-(1®4)-O-2,6-dideoxy-β-d-ribo-hexopyranosyl-(1®4)-2,6-dideoxy-β-d ribo-hexopyranosyl)oxy]-12β,14 dihydroxy-5β-card-20(22)-enolide. The molecular formula is C41H64O14 and molecular weight is 780.94.
STRUCTURAL FORMULA :
Its structural formula is :
DIGOXIN TABLETS USP is blue coloured, circular, biconvex uncoated tablets having score line on one side and ‘SGP’ embossed on the other side.
COMPOSITION :
Each uncoated tablet contains :
Digoxin USP 0.25 mg
Excipients q.s.
ACTIONS :
Digoxin increases contractility of the myocardium by direct activity. This effect is proportional to dose in the lower range and some effect is achieved with quite low dosing; it occurs even in normal myocardium although it is then entirely without physiological benefit. The primary action of digoxin is specifically to inhibit adenosine triphosphatase and thus sodium-potassium (Na+-K+) exchange activity, the altered ionic distribution across the membrane resulting in an augmented calcium ion influx and thus an increase in the availability of calcium at the time of excitation-contraction coupling. The potency of digoxin may therefore appear considerably enhanced when the extracellular potassium concentration is low, with hyperkalaemia having the opposite effect.
Digoxin exerts the same fundamental effect of inhibition of the Na+-K+ exchange mechanism on cells of the autonomic nervous system, stimulating them to exert indirect cardiac activity such as diminished impulse conduction rate through the atria and atrio-ventricular node (vagotonic) and senzitisation of the carotid sinus nerves (sympathomimetic). Indirect cardiac contractility changes also result from changes in venous compliance brought about by the altered autonomic activity and by direct venous stimulation. The interplay between direct and indirect activity governs the total circulatory response, which is not identical for all subjects. In the presence of certain supraventricular arrhythmias, the neurogenically mediated slowing of AV conduction is paramount.
The degree of neurohormonal activation occurring in patients with heart failure is associated with clinical deterioration and an increased risk of death. Digoxin reduces activation of both the sympathetic nervous system and the (renin-angiotensin) system independently of its inotropic actions and may favourably influence survival. Whether this is achieved via direct sympathoinhibitory effects or by re-sensitizing baroreflex mechanisms remains unclear.
PHARMACOKINETICS :
The absorption of digoxin from the gastrointestinal tract is variable depending upon the formulation used. About 70 % of a dose is absorbed from tablets which comply with B.P. or USP specifications. The generally accepted therapeutic plasma concentration range is 0.5 to 2.0 nanograms/ml but there is considerable inter individual variation. Digoxin has a large volume of distribution and is widely distributed in tissues, including the heart, brain, erythrocytes, and skeletal muscle. The concentration of digoxin in the myocardium is considerably higher than in plasma. From 20 to 30 % is bound to plasma proteins. Digoxin has been detected in cerebrospinal fluid (CSF) and breast milk; it also crosses the placenta. It has an elimination half-life of 1.5 to 2 days.
Digoxin is mainly excreted unchanged in the urine by glomerular filtration and tubular secretion; reabsorption also occurs. Extensive metabolism has been reported in a minority of patients. Excretion of digoxin is proportional to the glomerular filtration rate. After intravenous injection 50 to 70 % of the dose is excreted unchanged. Digoxin is not removed from the body by dialysis, and only small amounts are removed by exchange transfusion and during cardiopulmonary bypass.
INDICATIONS :
DIGOXIN TABLETS USP is indicated in :
DIGOXIN TABLETS USP is indicated in the management of chronic cardiac failure where the dominant problem is systolic dysfunction. Its therapeutic benefit is greatest in those patients with ventricular dilatation. DIGOXIN TABLETS USP is indicated in the management of chronic cardiac failure where the dominant problem is systolic dysfunction. Its therapeutic benefit is greatest in those patients with ventricular dilatation.
DIGOXIN TABLETS USP is indicated in the management of certain supraventricular arrhythmias, particularly atrial flutter and fibrillation, where a major beneficial effect is reduction of the ventricular rate.
Administration :
FOR ORAL USE.
Dosage :
Recommended dosages are average values that may require considerable modification because of individual sensitivity or associated conditions. Diminished renal function is the most important factor requiring modification of recommended doses.
In deciding the dose of digoxin, several factors must be considered :
1. The disease being treated. Atrial arrhythmias may require larger doses than heart failure.
2. The body weight of the patient. Doses should be calculated based upon lean or ideal body weight.
3. The patients renal function, preferably evaluated on the basis of creatinine clearance.
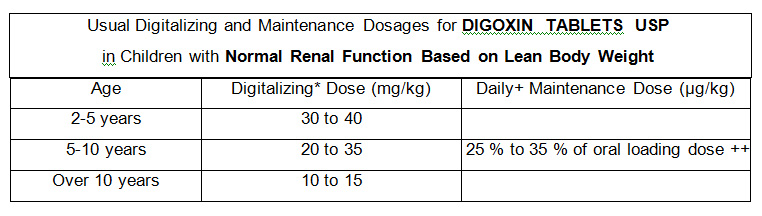
4. Age is an important factor in infants and children.
5. Concomitant disease states, drugs or other factors likely to alter the expected clinical response to digoxin.
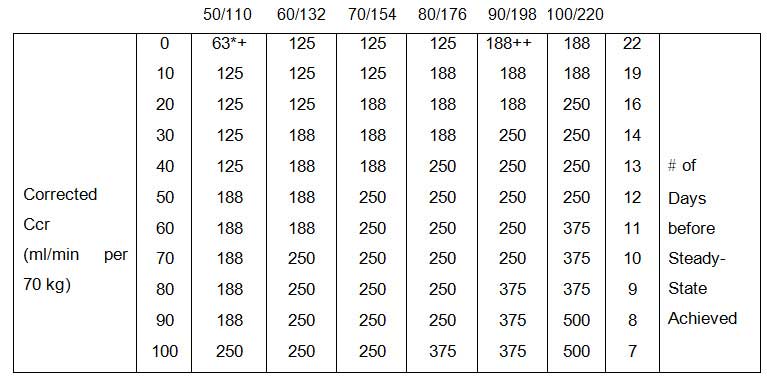
If a discrepancy exists between the reported serum concentration and the observed clinical response, the clinician should consider the following possibilities :
Arrhythmias may be precipitated by digoxin toxicity, some of which can resemble arrhythmias for which the drug could be advised. For example, atrial tachycardia with varying atrioventricular block requires particular care as clinically the rhythm resembles atrial fibrillation. Many beneficial effects of digoxin on arrhythmias result from a degree of atrioventricular conduction blockade. However, when incomplete atrioventricular block already exists, the effects of a rapid progression in the block should be anticipated. In complete heart block the idioventricular escape rhythm may be suppressed. In some cases of sinoatrial disorder (i.e. Sick Sinus Syndrome) digoxin may cause or exacerbate sinus bradycardia or cause sinoatrial block.
The administration of digoxin in the period immediately following myocardial infarction is not contra-indicated. However, the use of inotropic drugs in some patients in this setting may result in undesirable increases in myocardial oxygen demand and ischaemia, and some retrospective follow-up studies have suggested digoxin to be associated with an increased risk of death. The possibility of arrhythmias arising in patients who may be hypokalaemic after myocardial infarction and are likely to be haemodynamically unstable must be borne in mind. The limitations imposed thereafter on direct current cardioversion must also be remembered.
Treatment with digoxin should generally be avoided in patients with heart failure associated with cardiac amyloidosis. However, if alternative treatments are not appropriate, digoxin can be used to control the ventricular rate in patients with cardiac amyloidosis and atrial fibrillation. Digoxin can rarely precipitate vasoconstriction and therefore should be avoided in patients with myocarditis. Patients with beri beri heart disease may fail to respond adequately to digoxin if the underlying thiamine deficiency is not treated concomitantly. DIGOXIN TABLETS USP should not be used in constrictive pericarditis unless it is used to control the ventricular rate in arterial fibrillation or to improve systolic dysfunction. Digoxin improves exercise tolerance in patients with impaired left ventricular systolic dysfunction and normal sinus rhythm. This may or may not be associated with an improved haemodynamic profile. In patients receiving diuretics and an ACE inhibitor, or diuretics alone, the withdrawal of digoxin has been shown to result in clinical deterioration.
The use of therapeutic doses of digoxin may cause prolongation of the PR interval and depression of the ST segment on the electrocardiogram. Digoxin may produce false positive ST-T changes on the electrocardiogram during exercise testing. These electro physiologic effects reflect an expected effect of the drug and are not indicative of toxicity. In cases where cardiac glycosides have been taken in the preceding two weeks the recommendations for initial dosing of a patient should be reconsidered and a reduced dose is advised. The dosing recommendations should be reconsidered if patients are elderly or there are other reasons for the renal clearance of digoxin being reduced. A reduction in both initial and maintenance doses should be considered. Patients receiving DIGOXIN TABLETS USP should have their serum electrolytes and renal function (serum creatinine concentration) assessed periodically; the frequency of assessments will depend on the clinical setting.
Direct current cardioversion :
The risk of provoking dangerous arrhythmias with direct current cardioversion is greatly increased in the presence of digitalis toxicity and is in proportion to the cardioversion energy used. For elective direct current cardioversion of a patient who is taking digoxin, the drug should be withheld for 24 hours before cardioversion is performed. In emergencies, such as cardiac arrest, when attempting cardioversion the lowest effective energy should be applied. Direct current cardioversion is inappropriate in the treatment of arrhythmias thought to be caused by cardiac glycosides. DIGOXIN TABLETS USP should be used cautiously in diabetic patients.
Laboratory Tests :
Patients receiving digoxin should have their serum electrolytes and renal function (BUN and/or serum creatinine) assessed periodically; the frequency of assessments will depend on the clinical setting.
Pregnancy : Category C
Teratogenic Effects :
Animal reproduction studies have not been conducted with digoxin. It is also not known whether digoxin can cause foetal harm when administered to a pregnant woman or can affect reproductive capacity. Digoxin should be given to a pregnant woman only if clearly needed.
Nursing mothers :
Studies have shown that digoxin concentrations in the mother’s serum and milk are similar. However, the estimated exposure of a nursing infant to digoxin via breastfeeding will be far below the usual infant maintenance dose. Therefore, this amount should have no pharmacologic effect upon the infant. Nevertheless, caution should be exercised when digoxin is administered to a nursing woman.
Paediatric Use :
Newborn infants display considerable variability in their tolerance to digoxin. Premature and immature infants are particularly sensitive to the effects of digoxin, and the dosage of the drug must not only be reduced but must be individualized according to their degree of maturity. Digitalis glycosides can cause poisoning in children due to accidental ingestion.
INTERACTIONS AND INCOMPATIBILITIES :
Potassium-depleting diuretics are a major contributing factor to digitalis toxicity. Calcium, particularly if administered rapidly by the intravenous route, may produce serious arrhythmias in digitalized patients. Quinidine, verapamil, amiodarone, propafenone, indomethacin, itraconazole, alprazolam, and spironolactone raise the serum digoxin concentration due to a reduction in clearance and/or volume of distribution of the drug, with the implication that digitalis intoxication may result. Erythromycin and clarithromycin (and possibly other macrolide antibiotics) and tetracycline may increase digoxin absorption in patients who inactivate digoxin by bacterial metabolism in the lower intestine, so that digitalis intoxication may result. Propantheline and diphenoxylate, by decreasing gut motility, may increase digoxin absorption. Antacids, kaolin-pectin, sulfasalazine, neomycin, cholestyramine, certain anticancer drugs, and metoclopramide may interfere with intestinal digoxin absorption, resulting in unexpectedly low serum concentrations. Rifampin may decrease serum digoxin concentration, especially in patients with renal dysfunction, by increasing the non-renal clearance of digoxin. There have been inconsistent reports regarding the effects of other drugs (e.g., quinine, Penicillamine) on serum digoxin concentration. Thyroid administration to a digitalized, hypothyroid patient may increase the dose requirement of digoxin. Concomitant use of digoxin and sympathomimetics increases the risk of cardiac arrhythmias. Succinylcholine may cause a sudden extrusion of potassium from muscle cells, and may thereby cause arrhythmias in digitalized patients. Although calcium channel blockers and digoxin may be useful in combination to control atrial fibrillation, their additive effects on AV node conduction can result in advanced or complete heart block. Both digitalis glycosides and beta-blockers slow atrioventricular conduction and decrease heart rate. Concomitant use can increase the risk of bradycardia. Digoxin concentrations are increased by about 15 % when digoxin and carvedilol are administered concomitantly. Therefore, increased monitoring of digoxin is recommended when initiating, adjusting, or discontinuing carvedilol.
EFFECTS ON ABILITY TO DRIVE AND USE MACHINES :
Since central nervous system and visual disturbances have been reported in patients receiving DIGOXIN TABLETS USP, patients should exercise caution before driving, using machinery or participating in dangerous activities.
SIDE EFFECTS :
In general, the adverse reactions of digoxin are dose-dependent and occur at doses higher than those needed to achieve a therapeutic effect. Hence, adverse reactions are less common when digoxin is used within the recommended dose range or therapeutic serum concentration range and when there is careful attention to concurrent medications and conditions. Adverse reactions are listed below by system organ class and frequency. Frequencies are defined as : very common (≥1/10), common (≥1/100 and <1/10), uncommon (≥1/1000 and <1/100), rare (≥1/10,000 and <1/1000), very rare (1/10,000), including isolated reports. Very common, common and uncommon events were generally determined from clinical trial data. The incidence in placebo was taken into account. Adverse drug reactions identified through post-marketing surveillance were considered to be rare or very rare (including isolated reports).
Blood and lymphatic system disorders :
Very rare : Thrombocytopaenia
Metabolism and nutrition disorders :
Very rare : Anorexia
Psychiatric disorders :
Uncommon : Depression
Very rare : Psychosis, apathy, confusion.
Nervous system disorders :
Common : CNS disturbances, dizziness.
Very rare : Headache
Eye disorders :
Common : Visual disturbances (blurred or yellow vision)
Cardiac disorders :
Common : Arrhythmia, conduction disturbances, bigeminy, trigeminy, PR prolongation, sinus bradycardia.
Very rare : Supraventricular tachyarrhythmia, atrial tachycardia (with or without block), junctional (nodal) tachycardia, ventricular arrhythmia, ventricular premature contraction, ST segment depression.
Gastrointestinal disorders :
Common : Nausea, vomiting, diarrhoea.
Very rare : Intestinal ischaemia, intestinal necrosis.
Skin disorders :
Common : Skin rashes of urticarial or scarlatiniform character may be accompanied by pronounced eosinophilia.
Reproductive system and breast disorders :
Very rare : Gynaecomastia can occur with long term administration.
General disorders and administration site conditions :
Very rare : Fatigue, malaise, weakness.
Cardiac disorders :
Common : Arrhythmia, conduction disturbances, bigeminy, trigeminy, PR prolongation, sinus bradycardia.
Very rare : Supraventricular tachyarrhythmia, atrial tachycardia (with or without block), junctional (nodal) tachycardia, ventricular arrhythmia, ventricular premature contraction, ST segment depression.
Symptoms and signs :
The systems and signs of toxicity are generally similar to those described in the Side effects section but may be more frequent and can be more severe. Signs and symptoms of digoxin toxicity become more frequent with levels above 3.0 nanograms/ml (3.84 nanomol/l) although there is considerable interindividual variation. However, in deciding whether a patient’s symptoms are due to digoxin, the clinical state, together with serum electrolyte levels and thyroid function are important factors.

 Cardiovascular
Cardiovascular