1 mg
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
CABERGOLINE TABLETS USP (Cabergoline) is a long-acting, selective dopamine receptor agonist. Chemically, Cabergoline is 1-[(6-Allylergolin-8β-yl)carbonyl]-1-[3-(dimethylamino)propyl]-3-ethylurea. The molecular formula is C26H37N5O2 and the molecular weight is 451.60.
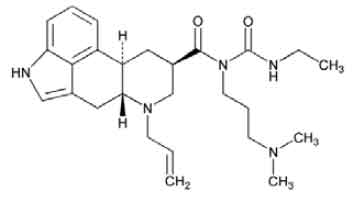
STRUCTURAL FORMULA :
Its structural formula is :
CABERGOLINE TABLETS USP are white coloured, circular biconvex uncoated tablets with breakline on one side and “SGP” embossed on other side.
COMPOSITION :
Each uncoated tablet contains :
Cabergoline USP 0.5 mg
Excipients q.s.
Each uncoated tablet contains :
Cabergoline USP 1 mg
Excipients q.s.
ACTIONS :
Cabergoline is a long-acting, selective dopamine receptor agonist, exhibiting high affinity for D2 receptors and low affinity for D1, alpha1- and alpha2-adrenergic, and serotonin (5-hydroxytryptamine1 and 5-hydroxytryptamine2) receptors. Cabergoline inhibits the synthesis and release of prolactin from the anterior pituitary by directly stimulating the D2 receptors of the pituitary Dactotrophs in a dose-related fashion. While cabergoline doses up to 2 mg inhibited prolactin in healthy volunteers, similar inhibition did not occur for the other anterior pituitary hormones, including growth hormone, follicle-stimulating hormone, luteinizing hormone, corticotropin, and thyroidstimulating hormone. Cabergoline did not affect serum cortisol concentrations.
PHARMACOKINETICS :
Absorption :
Exhibits first-pass effect; absolute bioavailability is unknown.
Distribution :
Extensive tissue distribution; cabergoline concentrations are at least 100 times greater in the pituitary than in the serum. Studies done in rats showed significant concentrations of cabergoline in the mammary glands and uterine wall, and that cabergoline crosses the placenta.
Protein binding :
Moderate (40 to 42 %), in a concentration-independent manner. Cabergoline’s protein binding is unlikely to be influenced by concomitant treatment with other protein-bound medications.
Biotransformation :
Hepatic - Cabergoline undergoes hydrolysis to inactive metabolites without causing hepatic enzyme induction or inhibition; cytochrome P450-mediated metabolism is minimal .Although mild to moderate hepatic function impairment does not alter pharmacokinetic values for cabergoline, severe hepatic function impairment (Child-Pugh score greater than 10) can substantially increase the values of Cmax and area under the plasma concentration-time curve (AUC).
Half-life :
Elimination - 63 to 69 hours.
Time to peak concentration : Within 3 hours.
Peak serum concentration : 30 to 70 picograms/mL (66.4 to 155 picomoles/L), reported in healthy volunteers taking single doses of 0.5 to 1.5 mg cabergoline. The steady-state serum concentration in patients using multiple weekly doses is expected to be 2 to 3 times higher than that reported for single doses.
Time to peak effect : 48 hours (single 0.6-mg dose of cabergoline in hyperprolactinaemic patients).
Duration of action : Up to 14 days (single 0.6-mg dose of cabergoline in hyperprolactinaemic patients).
Elimination : At 20 days for five healthy patients given single doses : Faecal : 60 %
Renal : 22 % (4 % unchanged)
Nonrenal clearance was 3.2 L per minute in healthy adults. Renal clearance was 0.08 L per minute, similar to that of hyperprolactinemic patients. Moderate-to-severe renal insufficiency did not alter cabergoline’s pharmacokinetics.
Administration :
CABERGOLINE TABLETS USP is given orally and should be taken with food.
Dosage :
Inhibition of physiological lactation :
CABERGOLINE TABLETS USP should be administered during the first day post-partum. The recommended therapeutic dose is 1 mg (two 0.5 mg tablets) as a single dose.
Treatment of hyperprolactinaemic disorders :
The recommended initial dosage of CABERGOLINE TABLETS USP is 0.5 mg per week given in one or two (½ of a 0.5 mg tablet) doses (e.g. on Monday and Thursday) per week. The weekly dose should be increased gradually preferably by adding 0.5 mg per week at monthly intervals until the optimal therapeutic response is achieved. The therapeutic dosage is usually 1 mg per week and ranges from 0.25 to 2 mg per week. Doses of CABERGOLINE TABLETS USP up to 4.5 mg per week have been used in hyperprolactinaemic patients.
In Parkinson’s disease :
CABERGOLINE TABLETS USP should be introduced gradually and during this period the dose of levodopa may be reduced gradually until an optimal response is achieved. A suggested initial dose of cabergoline given as a single daily dose is 0.5 mg in monotherapy or 1 mg in adjunctive therapy. The dose may be increased in increments of 0.5 or 1 mg at intervals of 7 or 14 days. CABERGOLINE TABLETS USP has recommended a maximum dose of 3 mg daily.
Severe hepatic insufficiency :
Lower doses should be considered in patients with severe hepatic insufficiency who receive prolonged treatment with cabergoline.
Children :
The safety and efficacy of cabergoline has not been established in subjects less than 16 years of age.
Elderly :
As a consequence of the indications for which cabergoline is presently proposed, the experience in the elderly is very limited. Available data do not indicate a special risk.
CONTRAINDICATIONS :
CABERGOLINE TABLETS USP are contraindicated in patients with
• Uncontrolled hypertension or known hypersensitivity to ergot derivatives.
• History of cardiac valvular disorders, as suggested by anatomical evidence of valvulopathy of any valve, determined by pre-treatment evaluation including echocardiographic demonstration of valve leaflet thickening, valve restriction, or mixed valve restriction-stenosis.
• History of pulmonary, pericardial, cardiac valvular, or retroperitoneal fibrotic disorders.
CABERGOLINE TABLETS USP contains lactose which is contra-indicated in patients with galactosaemia, the glucose-galactose malabsorption syndrome, or lactase deficiency.
WARNINGS :
General :
As with other ergot derivatives, CABERGOLINE TABLETS USP should be given with caution to patients with severe cardiovascular disease, Raynaud’s syndrome, peptic ulcer or gastrointestinal bleeding, or with a history of serious, particularly psychotic, mental disorders. Patients with rare hereditary problems of galactose intolerance, the Lapp lactase deficiency or glucose-galactose malabsorption should not take this medicine.
Postural Hypotension :
Postural hypotension can occur following administration of CABERGOLINE TABLETS USP , particularly during the first days of administration of CABERGOLINE TABLETS USP. Care should be exercised when administering CABERGOLINE TABLETS USP concomitantly with other drugs known to lower blood pressure.
Fibrosis and Cardiac Valvulopathy and Possibly Related Clinical Phenomena :
Fibrotic and serosal inflammatory disorders such as pleuritis, pleural effusion, pleural fibrosis, pulmonary fibrosis, pericarditis, pericardial effusion, cardiac valvulopathy involving one or more valves (aortic, mitral and tricuspid) or retroperitoneal fibrosis have occurred after prolonged usage of ergot derivatives with agonist activity at the serotonin 5HT2B receptor, such as CABERGOLINE TABLETS USP. In some cases, symptoms or manifestations of cardiac valvulopathy improved after discontinuation of CABERGOLINE TABLETS USP.
Erythrocyte sedimentation rate (ESR) has been found to be abnormally increased in association with pleural effusion/fibrosis. Chest x-ray examination is recommended in cases of unexplained ESR increases to abnormal values. Serum creatinine measurements can also be used to help in the diagnosis of fibrotic disorder. Following diagnosis of pleural effusion/pulmonary fibrosis or valvulopathy, the discontinuance of CABERGOLINE TABLETS USP has been reported to result in improvement of signs and symptoms. Valvulopathy has been associated with cumulative doses, therefore patients should be treated with the lowest effective dose. At each visit, the risk benefit profile of CABERGOLINE TABLETS USP treatment for the patient should be reassessed to determine the suitability of continued
treatment with CABERGOLINE TABLETS USP.
Before initiating long-term treatment :
All patients must undergo a cardiovascular evaluation, including echocardiogram, to assess the potential presence of asymptomatic valvular disease. It is also appropriate to perform baseline investigations of erythrocyte sedimentation rate or other inflammatory markers, lung function/chest x-ray and renal function prior to initiation of therapy. In patients with valvular regurgitation, it is not known whether CABERGOLINE TABLETS USP treatment might worsen the underlying disease. If fibrotic valvular disease is detected, the patient should not be treated with CABERGOLINE TABLETS USP.
CABERGOLINE TABLETS USP should be discontinued if an echocardiogram reveals new or worsened valvular regurgitation, valvular restriction or valve leaflet thickening. The need for other clinical monitoring (e.g., physical examination including, cardiac auscultation, X-ray, CT scan) should be determined on an individual basis. Additional appropriate investigations such as erythrocyte sedimentation rate, and serum creatinine measurements should be performed if necessary to support a diagnosis of a fibrotic disorder.
PRECAUTIONS :
General :
Initial doses higher than 1 mg may produce orthostatic hypotension. Care should be exercised when administering CABERGOLINE TABLETS USP with other medications known to lower blood pressure.
Postpartum Lactation Inhibition or Suppression :
CABERGOLINE TABLETS USP are not indicated for the inhibition or suppression of physiologic lactation. Use of bromocriptine, another dopamine agonist for this purpose, has been associated with cases of hypertension, stroke, and seizures.
Hepatic Impairment :
Since cabergoline is extensively metabolized by the liver, caution should be used, and careful monitoring exercised, when administering CABERGOLINE TABLETS USP to patients with hepatic impairment.
Psychiatric :
Pathological gambling, increased libido, and hypersexuality have been reported in patients treated with dopamine agonists including cabergoline. This has been generally reversible upon reduction of the dose or treatment discontinuation. CABERGOLINE TABLETS USP should be used cautiously in diabetic patients.
Pregnancy : Category B
Adequate and well-controlled studies in humans have not been done. Cabergoline is not recommended for use during pregnancy. Cabergoline crosses the placenta in animals. Researchers studied cabergoline effect on reproduction in mice, rats, and rabbits. Maternotoxicity, but not teratogenicity, occurred in studies of mice given doses of cabergoline 55 times greater than the MRHD (based on body surface area of a 50-kg human). When given doses of cabergoline equal to one seventh of the MRHD, rats experienced embryofoetal loss after embryo implantation. Similar studies in rabbits given doses 19 times greater than the MRHD produced maternotoxicity, exhibited as reduced food intake and body weight loss. Doses 150 times greater than the MRHD produced foetal malformations in rabbits in one study, a result not reproduced in another study using doses 300 times greater than the MRHD. The relevance of these findings to humans is not clear since prolactin affects the reproductive cycles of animals and humans differently.
Nursing Mothers :
It is not known if cabergoline is distributed into breast milk. Cabergoline should not be used in breast-feeding women or women planning to begin breast-feeding within a short period of time since it inhibits lactation by suppressing prolactin release. In a study in rats, continued treatment of female rats with cabergoline beginning 6 days before parturition resulted in arrested pup growth and death of the litter due to the decreased amount of available maternal milk.
Paediatric Use :
Appropriate studies on the relationship of age to the effects of cabergoline have not been performed in the paediatric population. Safety and efficacy have not been established.
EFFECTS ON ABILITY TO DRIVE AND USE MACHINES :
Patients being treated with CABERGOLINE TABLETS USP and presenting with somnolence and/or sudden sleep onset episodes must be informed to refrain from driving or engaging in activities where impaired alertness may put themselves or others at risk of serious injury or death (e.g. operating machines) until such episodes and somnolence have resolved.
INTERACTIONS :
The concomitant use of antiparkinson non-dopamine agonists (e.g. selegiline, amantadine, biperiden, trihexyphenidyl) was allowed in clinical studies for patients receiving CABERGOLINE TABLETS USP. In studies where the pharmacokinetic interactions of
CABERGOLINE TABLETS USP with L-dopa or selegiline were evaluated, no interactions were observed. No information is available about interaction between cabergoline and other ergot alkaloids : therefore the concomitant use of these medications during long-term treatment with CABERGOLINE TABLETS USP is not recommended. Since CABERGOLINE TABLETS USP exerts its therapeutic effect by direct stimulation of dopamine receptors, it should not be concurrently administered with drugs which have dopamine-antagonist activity (such as phenothiazines, butyrophenones, thioxanthenes, metoclopramide) since these might reduce the therapeutic effect of cabergoline. As with other ergot derivatives, CABERGOLINE TABLETS USP should not be used in association with macrolide antibiotics (e.g. erythromycin) due to increased systemic bioavailability.
SIDE EFFECTS :
Nausea, constipation, and headache are common side-effects of cabergoline. Paraesthesia has been reported rarely. Other reported side-effects include hypotension, dyskinesia, pathological gambling, increased libido, hypersexuality, leg cramps, allergic skin reactions, alopecia, and peripheral oedema. Cabergoline have been associated with pleuritis, pleural effusion, cardiac valvulopathy, pericardial effusion, constrictive pericarditis, and retroperitoneal, pleural, and pulmonary fibrosis. Cabergoline also reported drowsiness, dyspepsia, gastritis, epigastric and abdominal pain, angina, syncope, depression, confusion, hallucinations, breast pain; rarely vomiting, palpitation, epistaxis, digital vasospasm, hot flushes, transient hemianopia, muscle weakness; also reported cardiac valvulopathy, erythromelalgia.
INFORMATION FOR PATIENTS :
Patients should be instructed to notify their physician if they suspect they are pregnant, become pregnant, or intend to become pregnant during therapy. A pregnancy test should be done if there is any suspicion of pregnancy and continuation of treatment should be
discussed with their physician. Patients should notify their physician if they develop shortness of breath, persistent cough, difficulty with breathing when lying down, or swelling in their extremities.
OVERDOSAGE :
Symptoms :
Symptoms of overdose would likely be those of over-stimulation of dopamine receptors, e.g. nausea, vomiting, gastric complaints, postural hypotension, confusion/psychosis or hallucinations.
TREATMENT OF OVERDOSAGE :
Supportive measures should be taken to remove unabsorbed drug and maintain blood pressure, if necessary. In addition, the administration of dopamine antagonist drugs is advisable.
STORAGE :
Store at controlled room temperature 15°-30°C (59°-86°F), protected from moisture and light.
Do not refrigerate.
SHELF LIFE :
24 months from the date of manufacture.
PRESENTATION :
CABERGOLINE TABLETS USP contains Cabergoline USP 0.5 mg / 1 mg.
2 Blisters of 10 Tablets per Box.
Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.

 Cardiovascular
Cardiovascular