60 mg/2 ml
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
SOTRACIL (Sodium Tetradecyl Sulphate) is an anionic surfactant. Chemically, Sodium Tetradecyl Sulphate is all-rac-4-ethyl-1-isobutyloctyl sulphate. The molecular formula is C14H29NaO4S and molecular weight is 316.4.
STRUCTURAL FORMULA :
Its structural formula is :
-Structure.jpg)
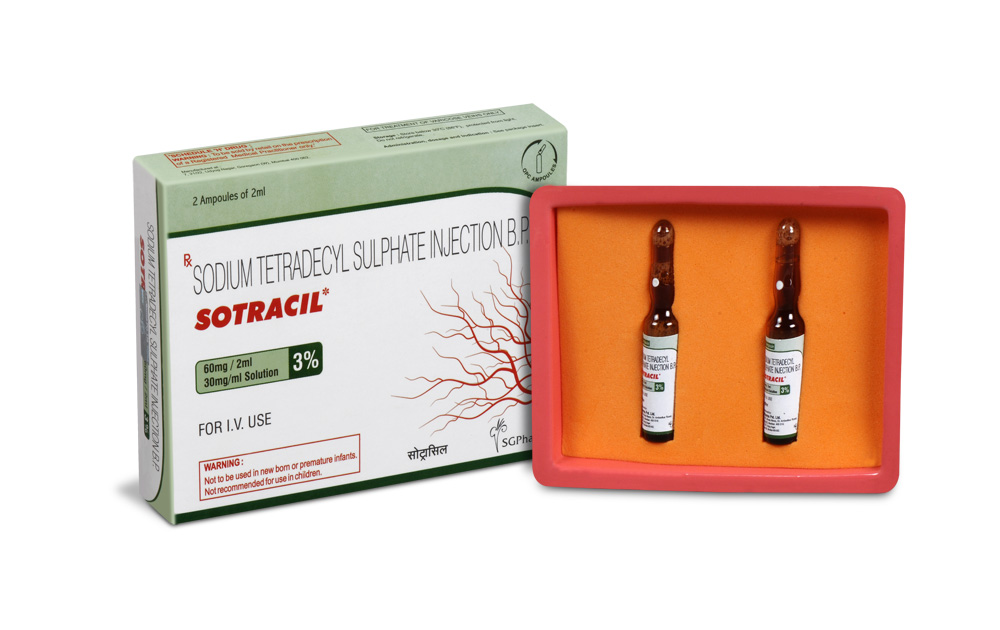
SOTRACIL is clear, colourless sterile aqueous solution filled in 2 ml amber glass ampoule.
COMPOSITION :
Each ml contains :
Sodium Tetradecyl Sulphate Concentrate
equivalent to Sodium Tetradecyl Sulphate 30 mg
Benzyl Alcohol I.P. 2 % v/v
(as preservative)
Water for Injections I.P. q.s.
ACTIONS :
Sodium Tetradecyl Sulphate is a sclerosing agent. Intravenous injection causes intima inflammation and thrombus formation. This usually occludes the injected vein. Subsequent formation of fibrous tissue results in partial or complete vein obliteration that may or may not be permanent.
INDICATIONS :
SOTRACIL is indicated in the treatment of small uncomplicated varicose veins of the lower extremities that show simple dilation with competent valves. The benefit-to-risk ratio should be considered in selected patients who are at great surgical risks.
Administration :
For intravenous administration into the lumen of an isolated segment of emptied vein followed by immediate continuous compression.
Compression Sclerotherapy :
The treatment of varicose veins by compression sclerotherapy is directed towards the restoration of the efficiency of the synchronised pumping systems within the leg by permanently destroying the leaking points rather than by the eradication of the superficial tortuous veins which may, in many cases, be capable of reverting to normal appearance and function after the restoration of the normal pattern of pressure within the veins of the limb. Localisation of the perforating veins containing the injured valves is the supremely important object of diagnosis.Treatment comprises the permanent blocking of the offending leak by producing a short fibrotic segment of vein involving the area of the junction of the perforating and superficial veins. This can be achieved by carrying out the following procedure :
1. Introducing the sclerosant into this vein after it has been emptied. Extreme care in the needle placement and slow injection of minimal effective volume at each injection site are essential for safe and efficient use.
2. Maintaining the sclerosant in the empty and isolated segment for 30 seconds.
3. Applying compression immediately to the site of injection, maintaining it for a period of about 6 weeks, until one is quite sure that, when the patient stands erect, the internal pressure of the blood in the adjacent unobliterated vein cannot reopen the segment.
4. Applications of compression is most suitable obtained by firm bandaging with a number of strong cotton crepe bandages and by incorporating therein shaped rubber pads over the sites of injection. An elastic stocking applied over the bandages aids compression and the retention of bandages in position. The use of a small dose, the isolation of the injection within the vein segment and the application of immediate, adequate and lasting compression are of supreme importance in obtaining a good result.
INSTRUCTIONS FOR USE OF AMPOULE :
The ampoule used in this product is equipped with O.P.C (One Point Cut) opening system. No ampoule file is needed to open the ampoule. The neck of the ampoule is prescored at the point of constriction. A coloured dot on the ampoule head helps to orientate the ampoule. Take the ampoule and face the coloured dot. Let the solution at the head of the ampoule to flow down by shaking or a gentle stroke. The ampoule opens easily by placing the thumb on the coloured dot and gently pressing downwards as shown.

Dosage :
A dose of 0.25 - 1 ml is injected intravenously into the lumen of an isolated segment of emptied superficial vein. This is followed by immediate continuous compression. It is never necessary or desirable to inject more than 1 ml at any one site and often half this volume will produce the desired effect. A maximum of four sites (4 ml total) may be injected during one treatment session.
CONTRAINDICATIONS :
The use of SOTRACIL is not recommended for the treatment of varicose veins by compression sclerotherapy when any of the following factors are present :
i) Oral Contraceptives :
Until such time as the exact thromboplastic effect of the oral contraceptive tablet has been established, it is advisable not to use sclerotherapy on patients who are currently taking oral contraceptives.
ii) Inability to walk :
As the recommended treatment involves daily periods of walking by the patient it is not advisable to embark upon treatment if for any reason the patient cannot walk at least 4½ kilometres each day.
iii) Obese legs :
It is recommended that patients with obese legs should not receive treatment. In many cases this obesity may be corrected by dieting, after which time treatment may be commenced.
iv) Any known allergy to Sodium Tetradecyl Sulphate :
Treatment by injection of SOTRACIL should not be continued if an allergic reaction has been experienced after a previous injection of Sodium Tetradecyl Sulphate.
v) Acute superficial thrombophlebitis or arterial disease :
SOTRACIL should not be used where there is thrombosis of the deep veins of the leg, acute phlebitis or other affections in the region of the varices.
vi) Local or systemic infection.
vii) Varicosities caused by pelvic or abdominal tumours.
viii) Uncontrolled systemic disease e.g. diabetes mellitus, toxic hyperthyroidism, tuberculosis, asthma, neoplasm, sepsis, blood dyscrasias and acute respiratory or skin disease.
ix) Significant valvular incompetence requiring surgical treatment.
WARNINGS :
Not recommended for use in children.
SOTRACIL should only be administered by a physician familiar with venous anatomy and the diagnosis and treatment of conditions affecting the venous system and familiar with proper injection technique. Severe adverse local effects, including tissue necrosis, may occur following extravasation therefore, extreme care in intravenous needle placement and using the minimal effective volumes at each injection site are important. Emergency resuscitation equipment should be immediately available. Allergic reactions, including fatal anaphylaxis, have been reported. As a precaution against anaphylactic shock, it is recommended that 0.5 ml of SOTRACIL be injected into a varicosity, followed by observation of the patient for several hours before administration of a second or larger dose. The possibility of an anaphylactic reaction should be kept in mind, and the physician should be prepared to treat it appropriately.
Equipment for treatment of anaphylaxis : The treatment of anaphylaxis may require, depending on severity of attack, some or all of the following : Injection of adrenaline, Injection of hydrocortisone, Antihistamine injection, Endotracheal tube, laryngoscope, Mucus extraction pump. The treatment of varicose veins by SOTRACIL should not be undertaken if these items are not readily available. Because of the danger of thrombosis extension into the deep venous system, thorough preinjection evaluation for valvular competency should be carried out and slow injections with a small amount (not over 2 ml) of the preparation should be injected into the varicosity. Deep venous patency must be determined by angiography or noninvasive testing such as duplex ultrasound. Venous sclerotherapy should not be undertaken if tests such as Trendelenberg and Perthes, and angiography show significant valvular or deep venous incompetence. The development of deep vein thrombosis and pulmonary embolism have been reported following sclerotherapy treatment of superficial varicosities. Patients should have post-treatment follow-up of sufficient duration to assess for the development of deep vein thrombosis. Embolism may occur as long as four weeks after injection of Sodium Tetradecyl Sulphate. Adequate post-treatment compression may decrease the incidence of deep vein thrombosis. This product contains benzyl alcohol as preservative. Benzyl alcohol has been reported to be associated with a fatal “Gasping Syndrome” in premature infants. Symptoms include a striking onset of gasping syndrome, hypotension, bradycardia and cardiovascular collapse.
PRECAUTIONS :
General :
Extreme caution must be exercised in the presence of underlying arterial disease such as marked peripheral arteriosclerosis or thromboangiitis obliterans (Buergers Disease).
Pregnancy :
Teratogenic Effects - Pregnancy Category C.
Animal reproduction studies have not been conducted with SOTRACIL. It is also not known whether SOTRACIL can cause foetal harm when administered to a pregnant woman or can affect reproduction capacity. SOTRACIL should be given to a pregnant woman only if clearly needed and the benefits outweigh the risks.
Nursing mothers :
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when SOTRACIL is administered to a nursing woman.
Paediatric Use :
Safety and effectiveness in paediatric patients have not been established. SOTRACIL is not recommended for children.
INTERACTIONS AND INCOMPATIBILITIES :
No well-controlled studies have been performed on patients taking antiovulatory agents. The physician must use judgement and evaluate any patient taking antiovulatory drugs prior to initiating treatment with SOTRACIL. Heparin should not be included in the same syringe as SOTRACIL, since the two are incompatible.
SIDE EFFECTS AND SPECIAL PRECAUTIONS :
Allergic reactions with SOTRACIL may occur. They may present as local or generalised rash, urticaria, nausea and vomiting, asthma or vascular collapse (anaphylaxis). Extravasation will give rise to pain and may produce necrosis and ulceration (if close to surface). Paraesthesia and anaesthesia may occur if an injection affects a cutaneous nerve. Pigmentation is more likely to occur if blood is extravasated at the injection site (especially when treating smaller veins and compression is not used). SOTRACIL should only be administered by practitioners familiar with an acceptable injection technique. Thorough pre-injection assessment for valvular competence and deep vein patency must be carried out. A history of allergy should be taken from all patients prior to treatment. Where special caution is indicated a test dose of 0.25 to 0.5 ml SOTRACIL should be given up to 24 hours before any further therapy. Special care is required when injecting above and posterior to the medial malleolus where the posterior tibial artery may be at risk.
OVERDOSAGE :
Symptoms of overdosage include those given in side effects and special precautions above.
TREATMENT OF OVERDOSAGE :
Treatment is supportive and symptomatic.
PHARMACEUTICAL PRECAUTIONS :
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
STORAGE :
Store below 30°C (86°F), protected from light.
Do not refrigerate.
SHELF LIFE :
24 months from the date of manufacture.
PRESENTATION :
SOTRACIL is aqueous solution supplied in 2 ml ampoule. Each ml contains Sodium Tetradecyl Sulphate 30 mg.
Such 2 ampoules of 2 ml are packed in a box.
Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.

 Cardiovascular
Cardiovascular