40 mg/ml
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
DOCETAXONE (Docetaxel) is a new semisynthetic analogue of Paclitaxel with a promising antitumour activity. Chemically, docetaxel trihydrate is (2R,3S)-N-Carboxy-3-phenylisoserine, N-tert-butyl ester, 13-ester with 5b-20-epoxy-1,2a,4,7b,10b,13a-hexahydroxytax-11-en-9-one 4-acetate 2-benzoate, trihydrate. The molecular formula is C43H53NO14.3H2O and molecular weight is 861.90.
STRUCTURAL FORMULA :
Its structural formula is :
-Structure.jpg)
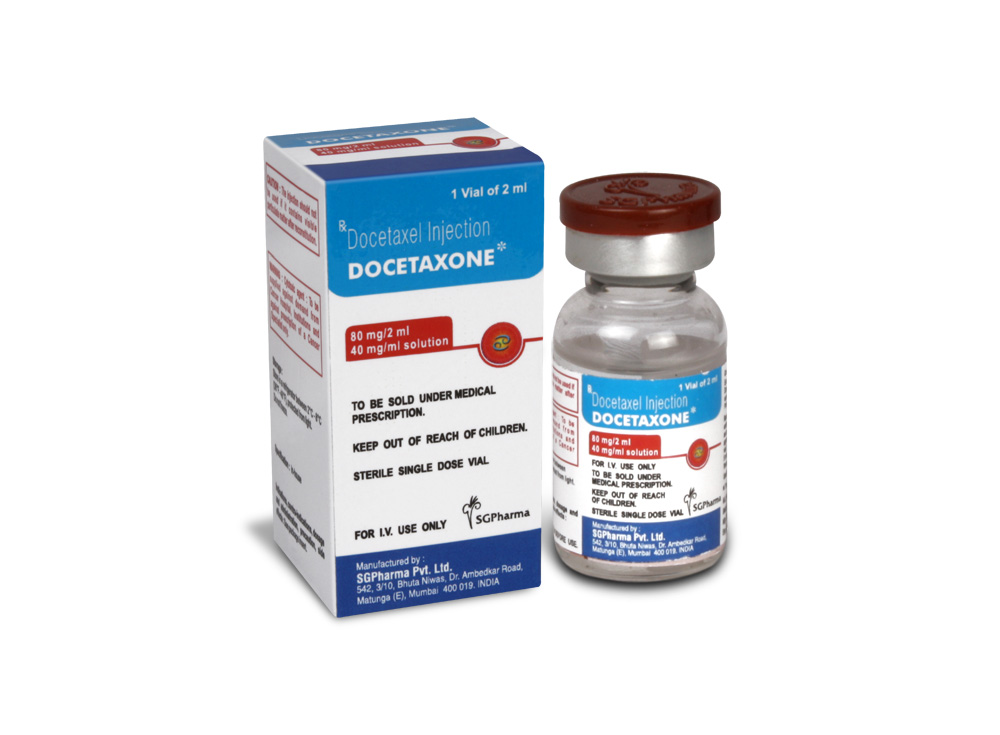
DOCETAXONE is a clear yellow to brownish-yellow viscous solution filled in vial of suitable size.
COMPOSITION :
Each ml contains :
Docetaxel Trihydrate I.P.
equivalent to anhydrous Docetaxel 40 mg
Polysorbate 80 I.P. q.s.
ACTIONS :
Docetaxel is an antineoplastic agent that acts by disrupting the microtubular network in cells that is essential for mitotic and interphase cellular functions. Docetaxel binds to free tubulin and promotes the assembly of tubulin into stable microtubules while simultaneously inhibiting their disassembly. This leads to the production of microtubule bundles without normal function and to the stabilization of microtubules, which results in the inhibition of mitosis in cells. Docetaxel’s binding to microtubules does not alter the number of protofilaments in the bound microtubules, a feature which differs from most spindle poisons currently in clinical use.
PHARMACOKINETICS :
The pharmacokinetic studies on docetaxel reveal that there is no schedule dependence of docetaxel disposition. The AUC increases in proportion to the dose of docetaxel administered, from 0.96 µg.h/ml (20 mg/m2) to 5.2 µg.h/ml (115 mg/m2), consistent with linear pharmacokinetics. At the highest clinical dose levels examined in the 1-h infusion schedule every 3 weeks, docetaxel disposition was triphasic with a terminal half-life of 11 to 18 h and a plasma clearance of 16 to 21 l/h/m2. Following the administration of 70-100 mg dose given as a 1-2 hour infusion, the peak plasma level of 3.8 µg/ml was obtained with a corresponding AUC of 4.7 h.µg/ml. The half lives for α, β and γ phases were 5 minutes, 36 minutes and 12.2 hours respectively while systemic clearance was 21 l/h/m2. On intravenous dosage docetaxel is rapidly distributed to body tissues. Docetaxel is more than 95 % bound to plasma proteins. It is extensively metabolised via hepatic cytochrome P450 isoenzyme CYP3A and excreted chiefly in the faeces as metabolites. Only about 6 % of a dose is excreted in urine. The terminal elimination half-life is about 11 hours. Clearance is reduced in hepatic impairment.
INDICATIONS :
Breast cancer :
DOCETAXONE in combination with doxorubicin and cyclophosphamide is indicated for the adjuvant treatment of patients with operable node-positive breast cancer.
DOCETAXONE in combination with doxorubicin is indicated for the treatment of patients with locally advanced or metastatic breast cancer who have not previously received cytotoxic therapy for this condition.
DOCETAXONE monotherapy is indicated for the treatment of patients with locally advanced or metastatic breast cancer after failure of cytotoxic therapy. Previous chemotherapy should have included an anthracycline or an alkylating agent.
DOCETAXONE in combination with trastuzumab is indicated for the treatment of patients with metastatic breast cancer whose tumors overexpress HER2 and who previously have not received chemotherapy for metastatic disease.
DOCETAXONE in combination with capecitabine is indicated for the treatment of patients with locally advanced or metastatic breast cancer after failure of cytotoxic chemotherapy. Previous therapy should have included an anthracycline.
Non-small cell lung cancer :
DOCETAXONE is indicated for the treatment of patients with locally advanced or metastatic non-small cell lung cancer after failure of prior chemotherapy. DOCETAXONE in combination with cisplatin is indicated for the treatment of patients with unresectable, locally advanced or metastatic non-small cell lung cancer, in patients who have not previously received chemotherapy for this condition.
Prostate cancer :
DOCETAXONE in combination with prednisone or prednisolone is indicated for the treatment of patients with hormone refractory metastatic prostate cancer.
Gastric adenocarcinoma :
DOCETAXONE in combination with cisplatin and 5-fluorouracil is indicated for the treatment of patients with metastatic gastric adenocarcinoma, including adenocarcinoma of the gastroesophageal junction, who have not received prior chemotherapy for metastatic disease.
Head and neck cancer :
DOCETAXONE in combination with cisplatin and 5-fluorouracil is indicated for the induction treatment of patients with locally advanced squamous cell carcinoma of the head and neck.
Administration :
DOCETAXONE should be administered by intravenous infusion only.
DIRECTIONS FOR USE :
Premedication :
In order to reduce adverse reactions like fluid retention, it is recommended that every patient should be premedicated with oral corticosteroids such as Dexamethasone 16 mg per day for 4-5 days starting 1 day before the administration of docetaxel. Docetaxel should not be administered until the neutrophil count is at least 1500 cell/mm3. Patients who experience either febrile neutropaenia, neutrophil < 500 cell/mm3 for more than one week, severe or cumulative cutaneous reactions or severe peripheral neuropathy during docetaxel therapy should have the dosage of docetaxel reduced from 100 mg/m2 to 75 mg/m2. If the patient continues to experience these reactions at 75 mg/m2, the dosage should be decreased from 75 mg/m2 to 55 mg/m2.
Handling and disposal :
Standard procedures for proper handling and disposal of anti-cancer drugs should be considered. Several guidelines have been issued but there is no general agreement that all the procedures are necessary or appropriate. However, the following general procedure may be adopted.
Preparation for the intravenous administration :
a) Preparation of DOCETAXONE premix solution :
Take out required number of vials of DOCETAXONE from the refrigerator and allow them to stand for 5 minutes to bring it to room temperature. Withdraw the entire contents of the solvent vial into a syringe and transfer aseptically to the vial of DOCETAXONE. Shake the vial for 15 seconds by manual rotation and then allow this DOCETAXONE premix solution to stand for 5 minutes at room temperature. The solution should be clear and homogenous. There may be foaming which is due to Polysorbate 80 I.P. The DOCETAXONE premix solution contains 10 mg docetaxel/ml.
b) Preparation of the infusion solution :
Withdraw aseptically the required amount of DOCETAXONE premix solution with a calibrated syringe and inject the premix volume into an infusion bag or bottle of either 0.9 % Sodium Chloride Injection I.P. or 5 % Dextrose Injection I.P. to produce a final concentration of 0.3 to 0.9 mg docetaxel/ml. The infusion solution should then be thoroughly mixed by manual rotation. Administer intravenously docetaxel infusion solution as one hour intravenous infusion under room temperature and normal lighting conditions. Do not admix with other medications.Docetaxel infusion is compatible with commonly available IV administration sets including PVC sets.
Dosage :
Breast cancer :
The recommended dose of DOCETAXONE is 60-100 mg/m2 administered intravenously over 1 hour every 3 weeks.
Non-small cell lung cancer :
For treatment after failure of prior platinum-based chemotherapy, DOCETAXONE was evaluated as monotherapy, and the recommended dose is 75 mg/m2 administered intravenously over 1 hour every 3 weeks. A dose of 100 mg/m2 in patients previously treated with chemotherapy was associated with increased haematologic toxicity, infection, and treatment-related mortality in randomized, controlled trials. For chemotherapy-naïve patients, DOCETAXONE was evaluated in combination with cisplatin. The recommended dose of DOCETAXONE is 75 mg/m2 administered intravenously over 1 hour immediately followed by cisplatin 75 mg/m2 over 30-60 minutes every 3 weeks.
Prostate cancer :
For hormone-refractory metastatic prostate cancer, the recommended dose of DOCETAXONE is 75 mg/m2 every 3 weeks as a 1 hour infusion. Prednisone 5 mg orally twice daily is administered continuously.
Premedication regimen :
All patients should be premedicated with oral corticosteroids such as dexamethasone 16 mg per day (e.g. 8 mg BID) for 3 days starting 1 day prior to DOCETAXONE administration in order to reduce the incidence and severity of fluid retention as well as the severity of hypersensitivity reactions. For hormone-refractory metastatic prostate cancer, given the concurrent use of prednisone, the recommended premedication regimen is oral dexamethasone 8 mg, at 12 hours, 3 hours and 1 hour before the DOCETAXONE infusion.
Dosage adjustments during treatment :
Breast cancer :
Patients who are dosed initially at 100 mg/m2 and who experience either febrile neutropaenia, neutrophils < 500 cells/mm3 for more than 1 week, or severe or cumulative cutaneous reactions during DOCETAXONE therapy should have the dosage adjusted from 100 mg/m2 to 75 mg/m2. If the patient continues to experience these reactions, the dosage should either be decreased from 75 mg/m2 to 55 mg/m2 or the treatment should be discontinued. Conversely, patients who are dosed initially at 60 mg/m2 and who do not experience febrile neutropaenia, neutrophils < 500 cells/mm3 for more than 1 week, severe or cumulative cutaneous reactions, or severe peripheral neuropathy during DOCETAXONE therapy may tolerate higher doses. Patients who develop >/= grade 3 peripheral neuropathy should have DOCETAXONE treatment discontinued entirely.
Non-small cell lung cancer :
Monotherapy with DOCETAXONE for NSCLC treatment after failure of prior platinum-based chemotherapy :
Patients who are dosed initially at 75 mg/m2 and who experience either febrile neutropaenia, neutrophils < 500 cells/mm3 for more than one week, severe or cumulative cutaneous reactions, or other grade 3/4 non-haematological toxicities during DOCETAXONE treatment should have treatment withheld until resolution of the toxicity and then resumed at 55 mg/m2 . Patients who develop >/= grade 3 peripheral neuropathy should have DOCETAXONE treatment discontinued entirely.
Combination therapy with DOCETAXONE for chemotherapy-naïve NSCLC :
For patients who are dosed initially at DOCETAXONE 75 mg/m2 in combination with cisplatin, and whose nadir of platelet count during the previous course of therapy is < 25,000 cells/mm3, in patients who experience febrile neutropaenia, and in patients with serious non-haematologic toxicities, the DOCETAXONE dosage in subsequent cycles should be reduced to 65 mg/m2 . In patients who require a further dose reduction, a dose of 50 mg/m2 is recommended.
Combination therapy with DOCETAXONE for hormone-refractory metastatic prostate cancer :
DOCETAXONE should be administered when the neutrophil count is >/=1,500 cells/mm3. Patients who experience either febrile neutropaenia, neutrophils < 500 cells/mm3 for more than one week, severe or cumulative cutaneous reactions or moderate neurosensory signs and/or symptoms during DOCETAXONE therapy should have the dosage of DOCETAXONE reduced from 75 to 60 mg/m2. If the patient continues to experience these reactions at 60 mg/m2, the treatment should be discontinued.
Special populations :
Hepatic impairment :
Patients with bilirubin > ULN should generally not receive DOCETAXONE. Also, patients with SGOT and/or SGPT > 1.5 × ULN concomitant with alkaline phosphatase > 2.5 × ULN should generally not receive DOCETAXONE.
Children : The safety and effectiveness of docetaxel in paediatric patients below the age of 16 years have not been established.
Elderly : In general, dose selection for an elderly patient should be cautious, reflecting the greater frequency of decreased hepatic, renal, or cardiac function and of concomitant disease or other drug therapy in elderly patients.
CONTRAINDICATIONS :
DOCETAXONE is contraindicated in patients who have a history of severe hypersensitivity reactions to docetaxel or to other drugs formulated with polysorbate 80.
DOCETAXONE should not be used in patients with neutrophil counts of < 1500 cells/mm3.
WARNINGS :
The incidence of treatment-related mortality associated with DOCETAXONE therapy is increased in patients with abnormal liver function, in patients receiving higher doses, and in patients with non-small cell lung carcinoma and a history of prior treatment with platinum-based chemotherapy who receive DOCETAXONE as a single agent at a dose of 100 mg/m2. DOCETAXONE should generally not be given to patients with bilirubin > upper limit of normal (ULN), or to patients with SGOT and/or SGPT > 1.5 × ULN concomitant with alkaline phosphatase > 2.5 × ULN. Patients with elevations of bilirubin or abnormalities of transaminase concurrent with alkaline phosphatase are at increased risk for the development of grade 4 neutropaenia, febrile neutropaenia, infections, severe thrombocytopaenia, severe stomatitis, severe skin toxicity, and toxic death. Patients with isolated elevations of transaminase > 1.5 × ULN also had a higher rate of febrile neutropaenia grade 4 but did not have an increased incidence of toxic death. Bilirubin, SGOT or SGPT, and alkaline phosphatase values should be obtained prior to each cycle of DOCETAXONE therapy and reviewed by the treating physician. DOCETAXONE therapy should not be given to patients with neutrophil counts of < 1500 cells/mm3. In order to monitor the occurrence of neutropaenia, which may be severe and result in infection, frequent blood cell counts should be performed on all patients receiving DOCETAXONE.
Severe hypersensitivity reactions characterized by generalized rash/erythema, hypotension and/or bronchospasm, or very rarely fatal anaphylaxis, have been reported in patients who received the recommended 3-day dexamethasone premedication. Hypersensitivity reactions require immediate discontinuation of the DOCETAXONE infusion and administration of appropriate therapy. DOCETAXONE must not be given to patients who have a history of severe hypersensitivity reactions to DOCETAXONE or to other drugs formulated with Polysorbate 80. Severe fluid retention occurred in 6.5 % (6/92) of patients despite use of a 3-day dexamethasone premedication regimen. It was characterized by one or more of the following events : poorly tolerated peripheral oedema, generalized oedema, pleural effusion requiring urgent drainage, dyspnoea at rest, cardiac tamponade, or pronounced abdominal distention (due to ascites).
Pregnancy : Category D
DOCETAXONE can cause foetal harm when administered to pregnant women. Studies in both rats and rabbits at doses >/= 0.3 and 0.03 mg/kg/day, respectively (about 1/50 and 1/300 the daily maximum recommended human dose on a mg/m2 basis), administered during the period of organogenesis, have shown that DOCETAXONE is embryotoxic and foetotoxic (characterized by intrauterine mortality, increased resorption, reduced foetal weight, and foetal ossification delay). The doses indicated above also caused maternal toxicity. There are no adequate and well-controlled studies in pregnant women using DOCETAXONE. If DOCETAXONE is used during pregnancy, or if the patient becomes pregnant while receiving this drug, the patient should be apprised of the potential hazard to the foetus or potential risk for loss of the pregnancy. Women of childbearing potential should be advised to avoid becoming pregnant during therapy with DOCETAXONE.
Nursing mothers :
It is not known whether DOCETAXONE is excreted in human milk. Because many drugs are excreted in human milk, and because of the potential for serious adverse reactions in nursing infants from DOCETAXONE, mothers should discontinue nursing prior to taking the drug.
Paediatric Use :
The safety and effectiveness of DOCETAXONE in paediatric patients have not been established.
INTERACTIONS AND INCOMPATIBILITIES :
In vitro studies have shown that the metabolism of docetaxel may be modified by the concomitant administration of compounds which induce, inhibit or are metabolised by (and thus may inhibit the enzyme competitively) cytochrome P450-3A such as ciclosporine, terfenadine, ketoconazole, erythromycin and troleandomycin. As a result, caution should be exercised when treating patients with these medicinal products as concomitant therapy since there is a potential for a significant interaction. Docetaxel is highly protein bound (> 95 %). Although the possible in vivo interaction of docetaxel with concomitantly administered medication has not been investigated formally, in vitro interactions with tightly protein-bound agents such as erythromycin, diphenhydramine, propranolol, propafenone, phenytoin, salicylate, sulfamethoxazole and sodium valproate did not affect protein binding of docetaxel. In addition, dexamethasone did not affect protein binding of docetaxel. Docetaxel did not influence the binding of digitoxin. The pharmacokinetics of docetaxel, doxorubicin and cyclophosphamide were not influenced by their coadministration. Limited data from a single uncontrolled study were suggestive of an interaction between docetaxel and carboplatin. When combined to docetaxel, the clearance of carboplatin was about 50 % higher than values previously reported for carboplatin monotherapy. Docetaxel pharmacokinetics in the presence of prednisone was studied in patients with metastatic prostate cancer. Docetaxel is metabolised by CYP3A4 and prednisone is known to induce CYP3A4. No statistically significant effect of prednisone on the pharmacokinetics of docetaxel was observed.
Docetaxel should be administered with caution in patients concomitantly receiving potent CYP3A4 inhibitors (e.g. protease inhibitors like ritonavir, azole antifungals like ketoconazole or itraconazole). A drug interaction study performed in patients receiving ketoconazole and docetaxel showed that the clearance of docetaxel was reduced by half by ketoconazole, probably because the metabolism of docetaxel involves CYP3A4 as a major (single) metabolic pathway. Reduced tolerance of docetaxel may occur, even at lower doses.
SIDE EFFECTS :
The following side effects have been reported from several clinical trials on docetaxel :
• Bone Marrow Suppression :
Usually mild but in some cases severe neutropaenia (neutrophils < 500 cell/mm3) has been reported. This is non-cumulative and reversible. Neutropaenic fever and anaemia have also been reported. Severe thrombocytopaenia has been reported in a few patients.
• Hypersensitivity reactions :
Mild hypersensitivity reactions such as flushing, tightness in the chest, rashes, pruritis, mild dyspnoea, or chills may occur. Severe hypersensitivity reactions in the form of hypotension (fall in B.P. by more than 20 mm Hg) and bronchospasm may occur. This may require discontinuation of therapy and aggressive symptomatic treatment.
• Fluid retention :
Gain of body weight by more than 3 kg has been reported after 4 or more cycles or after a cumulative dose of 400 mg/m2 as a result of fluid retention. Fluid retention in the form of oedema, pleural effusion, ascites and increased capillary permeability has been reported. Discontinuation of docetaxel treatment causes slow reversal of this fluid retention. Premedication with oral corticosteroids has been observed to reduce the fluid retention.
• Skin reaction :
Skin reaction has been observed in the form of rash, localized eruptions mainly on feet, hands, arms, face and chest and are often associated with itching. Usually these reactions occur within a week of docetaxel infusion and recover before the next infusion. Rarely severe symptoms such as desquamation may occur. Nails have also been reported to exhibit symptoms of toxicity as hyperpigmentation, pain and onycholysis.
• Gastrointestinal effects such as nausea, vomiting or diarrhoea may occur.
• Neurotoxicity has been reported during the clinical trials conducted abroad.
• Cardiovascular events of clinical meaning occurred rarely.
• Other undesirable effects have included alopecia, asthenia, mucositis, arthralgias.
OVERDOSAGE :
The known complications of overdose include neutropaenia, cutaneous reactions and sensory neuropathy.
TREATMENT OF OVERDOSAGE :
There is no known antidote for docetaxel overdose. The patient should be kept in a specialized unit and vital functions should be closely monitored in such cases of overdose and myalgias, hypotension and injection site reactions.
PHARMACEUTICAL PRECAUTIONS :
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
STORAGE :
Store under refrigeration between 2°C - 8°C (36°F - 46°F), protected from light.
Do not freeze.
SHELF LIFE :
24 months from the date of manufacture.
PRESENTATION :
DOCETAXONE (Docetaxel Injection Concentrate I.P.) is supplied as :
-Table.jpg)
Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.

 Cardiovascular
Cardiovascular