0.5 mg/ml
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
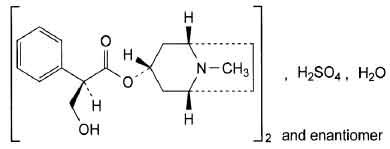
ATROPINE SULFATE INJECTION USP (Atropine Sulfate) is a parenteral anticholinergic agent and muscarinic antagonist. Chemically, (Atropine Sulfate) is Benzeneacetic acid, α-(hydroxymethyl)-,8-methyl-8-azabicyclo[3.2.1] oct-3-ylester,endo-(±)-, sulfate (2:1) (salt), monohydrate. The molecular formula is (C17H23NO3)2•H2SO4•H2O and molecular weight is 694.83.
STRUCTURAL FORMULA :
Its structural formula is :
ATROPINE SULFATE INJECTION USP is a clear, colourless, sterile solution filled in ampoule of suitable size.
COMPOSITION :
Each ml contains :
Atropine Sulfate USP 0.5 mg
Sodium Chloride USP 9 mg
Water for Injection USP q.s.
ACTIONS :
Atropine is often classified as an anticholinergic drug but is more accurately described as an antimuscarinic agent since it inhibits the muscarinic actions of acetylcholine, possessing both central and peripheral activity. Atropine has activity both on structures innervated by postganglionic cholinergic nerves, and on smooth muscles which respond to endogenous acetylcholine but are not so innervated. As with other antimuscarinic agents, the major action of atropine is a competitive or surmountable antagonism which can be overcome
by increasing the concentration of acetylcholine at receptor sites of the effector organ (e.g. by using anticholinesterase agents which inhibit the enzymatic destruction of acetylcholine). The receptors antagonised by atropine in therapeutic doses are primarily the peripheral structures that are stimulated or inhibited by muscarine (i.e. Exocrine glands and smooth and cardiac muscle). Responses to postganglionic cholinergic nerve solution also may be inhibited by atropine but this occurs less readily than with responses to injected (exogenous) choline esters. Atropine-induced parasympathetic inhibition may be preceded by a transient phase of stimulation, especially on the heart where small doses first slow the rate before characteristic tachycardia develops due to paralysis of vagal control. Atropine exerts a more potent and prolonged effect on the heart, intestine and bronchial muscle than hyoscine, but its action on the iris, ciliary body and certain secretory glands is weaker than that of hyoscine. Unlike the latter, atropine in therapeutic doses does not depress the central nervous system but may stimulate the medulla and higher cerebral centres. Although mild vagal excitation occurs, the increased respiratory rate and (sometimes) increased depth of respiration produced by atropine are more probably the result of bronchiolar dilatation. Accordingly, atropine is an unreliable respiratory stimulant and large or repeated doses may depress respiration.
Adequate doses of atropine abolish various types of reflex vagal cardiac slowing or asystole. The drug also prevents or abolishes bradycardia or asystole produced by injection of choline esters, anticholinesterase agents or other parasympathetic drugs, and cardiac arrest produced by stimulation of the vagus. Atropine sulfate injection in therapeutic doses counteracts the peripheral dilatation and abrupt decrease in blood pressure produced by choline esters. However, when given by itself, atropine does not exert a striking or uniform effect on blood vessels or blood pressure. Systemic doses slightly raise systolic and lower diastolic pressures and can produce significant postural hypotension. Such doses also slightly increase cardiac output and decrease central venous pressure. Occasionally, therapeutic doses dilate the cutaneous blood vessels, particularly in the “blush” area (atropine flush), and may cause atropine “fever” due to suppression of sweat gland activity in infants and small children.
PHARMACOKINETICS :
Absorption :
Atropine is well absorbed following intramuscular administration. Peak plasma levels are observed within 30 minutes of injection, accompanied by an increase in heart rate which reaches a maximum at 15 to 50 minutes. The duration of effect on the heart rate is reported to be up to 5 hours. The effect on salivation is delayed, with a peak effect occurring approximately 100 minutes after the injection and persisting for up to 4 hours.
Distribution :
Following intravenous administration serum levels of atropine drop rapidly within the first ten minutes and then decrease more gradually. One hour after either I.M. or I.V. injection atropine levels are very similar. The drug is well distributed throughout the body, crossing both the blood-brain and placental barriers, and distributing into the milk in small quantities. It has a large apparent volume of distribution (2 - 4 l/kg). Atropine shows a high interindividual variability in serum protein binding, ranging from 22.5 % ± 20.6 % (children); 14 % ± 9.1 % (age 16 to 58) ; 22.2 % ± 16.7 % (age 65 to 75).
Metabolism and Excretion :
Atropine is metabolised in the liver and excreted mainly in the urine. About 30 – 50 % is excreted unchanged in the urine. Atropine has a plasma half life of approximately 4 hours in adults, with a longer half-life of approximately 6.5 hours in children.
INDICATIONS :
Surgery :
ATROPINE SULFATE INJECTION USP may be given as a pre-anaesthetic medication to inhibit excessive salivary and bronchial secretions and to diminish the risk of vagal inhibition of the heart. The use of atropine as an antisialogogue is rarely necessary since the introduction of halothane and similar anaesthetics in place of ether anaesthesia. After surgery atropine may also be administered concurrently with anticholinesterase agents (e.g. neostigmine, physostigmine) when used to terminate curarisation to counteract the adverse muscarinic effects of these drugs.
Cardiopulmonary resuscitation :
Atropine may be used during cardiopulmonary resuscitation to treat sinus bradycardia and associated hypotension, and increased ventricular irritability.
Anticholinesterase poisoning :
ATROPINE SULFATE INJECTION USP is also used in the treatment of sinus bradycardia induced by organophosphate pesticides, Amanita muscaria mushrooms or other compounds with anticholinesterase activity. A cholinesterase reactivator, e.g. pralidoxime iodide, may be given concurrently.
Administration :
ATROPINE SULFATE INJECTION USP may be administered by subcutaneous (S.C.), intramuscular (I.M.) or direct intravenous (I.V) injection. ATROPINE SULFATE INJECTION USP should not be added to any I.V. infusion solution.
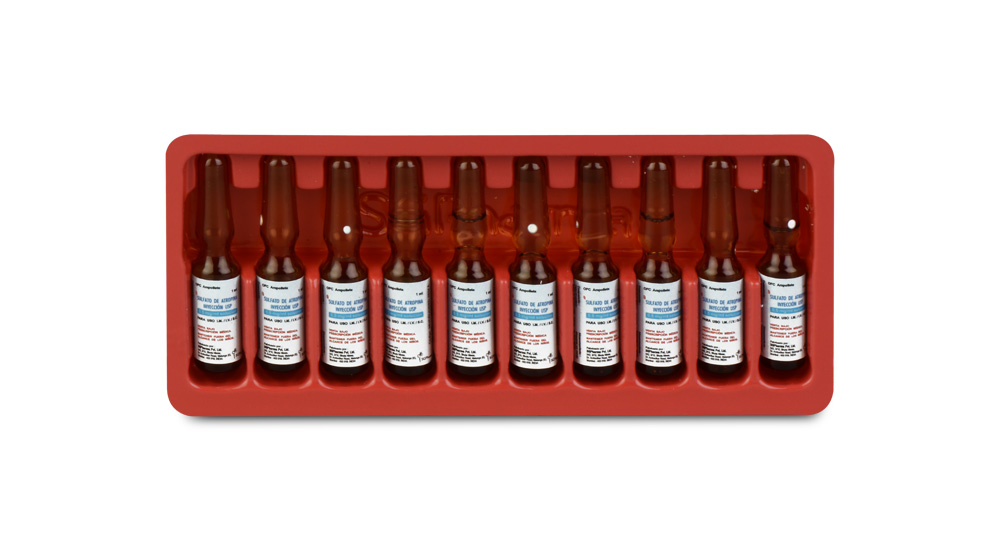
INSTRUCTIONS FOR USE OF AMPOULE :
The ampoule used in this product is equipped with O.P.C (One Point Cut) opening system. No ampoule file is needed to open the ampoule. The neck of the ampoule is prescored at the point of constriction. A coloured dot on the ampoule head helps to orientate the ampoule. Take the ampoule and face the coloured dot. Let the solution at the head of the ampoule to flow down by shaking or a gentle stroke. The ampoule opens easily by placing the thumb on the coloured dot and gently pressing downwards as shown.

Dosage :
Surgery
Adults :
300 - 600 µg of atropine sulfate I.M. or S.C., approximately 1 hour before anaesthesia, usually in conjunction with a narcotic. Alternatively, 300 - 600 µg atropine sulfate may be given I.V. immediately prior to induction of anaesthesia. To reverse the effects of non depolarising muscle relaxants, 600 µg - 1.2 mg atropine sulfate may be given to adults as a slow I.V. injection in conjunction with the anticholinesterase agent (e.g. neostigmine methylsulfate) of choice.
Paediatrics :
Suitable premedication doses to be given subcutaneously 30 – 60 minutes prior to surgery in children are suggested below :
Infants weighing less than 3 kg : 100 µg
Children weighing 7 to 9 kg : 200 µg
Children weighing 12 to 16 kg : 300 µg
Children weighing 20 to 27 kg : 400 µg
Children weighing 32 kg : 500 µg
Children weighing 41 kg : 600 µg.
To reverse the effects of non depolarising muscle relaxants, Atropine 0.02 mg/kg for each 0.04 mg/kg of neostigmine methylsulfate may be given to children as a slow I.V. injection.
Cardiopulmonary Resuscitation :
Adults :
Atropine sulfate 0.5 – 1 mg I.V. may be repeated every 5 minutes until the desired effect on heart rate or asystole is achieved. I.V. doses less than 0.5 mg should usually not be used in adults, since paradoxical slowing of the heart rate may occur. The total dose should not exceed 2 mg.
Paediatrics :
The usual paediatric dose is 0.02 mg/kg with a minimum of 0.1 mg (maximum single dose 0.5 mg) intravenously, which may be repeated at five minute intervals until the desired heart rate is achieved. The total dose should not exceed 1 mg in children and 2 mg in adolescents. When cardiac arrest has occurred, external cardiac massage or other method of resuscitation is required to distribute the drug after intravenous injection.
CONTRAINDICATIONS :
- Known hypersensitivity to atropine or other anticholinergic agents.
- Obstructive disease of the gastrointestinal tract (e.g. pyloroduo denal stenosis, bachalasia), cardiospasm, paralytic ileus or intestinal atony.
- Reflux oesophagitis.
- Severe ulcerative colitis or megacolon complicating ulcerative colitis.
- Prostatic enlargement.
- Acute angle-closure glaucoma.
- Unstable cardiovascular status in acute haemorrhage.
- Tachycardia secondary to cardiac insufficiency or thyrotoxicosis.
- Toxaemia of pregnancy.
- Obstructive uropathy.
- Myasthenia gravis (unless used to treat the adverse effects of an anticholinesterase agent).
- Due to the risk of provoking hyperpyrexia due to reduced sweating, atropine should not be used in febrile patients or when the ambient temperature is high.
WARNINGS :
ATROPINE SULFATE INJECTION USP should be used with caution in conditions characterised by tachycardia such as thyrotoxicosis, cardiac insufficiency or failure and in cardiac surgery. ATROPINE SULFATE INJECTION USP should be used only with extreme caution in toxic pyrexial children, or in high ambient temperatures, because of the danger of hyperpyrexia. When used to treat a cholinergic crisis in myasthenia gravis, all anticholinesterase medication should be withdrawn, if necessary for several days. Doses of Atropine up to 1 mg are mildly stimulant to the central nervous system. Higher doses may induce mental disturbances and depression of the central nervous system. Children and elderly people are particularly susceptible. Care is required when using atropine in the presence of acute myocardial ischaemia or infarction as the ischaemia or infarction may be worsened.
PRECAUTIONS :
ATROPINE SULFATE INJECTION USP should be used with caution in all patients, and especially in patients over 40 years old as they may be more susceptible to adverse effects. It should be administered with extreme care in patients with any severe heart disease, hypertension, mild or moderate ulcerative colitis, ileus, chronic pulmonary disease, hyperthyroidism, autonomic neuropathy, hepatic or renal disease or prostatic hypertrophy, oesophageal reflux or hiatus hernia, gastric ulcer, diarrhoea, or gastrointestinal infection.
Elderly patients may react with excitement, agitation, drowsiness or confusion to even small doses of atropine. Changes in dosage should be gradual.
Children :
ATROPINE SULFATE INJECTION USP should be used with caution in infants and small children as they may be more susceptible to its adverse effects. Children are particularly at risk of rapid increase in body temperature in hot weather, due to reduced sweating.
ATROPINE SULFATE INJECTION USP should be used with caution in children with Down’s syndrome, spastic paralysis or brain damage as they may be hypersensitive to the effects of atropine.
Debilitated and Elderly patients :
ATROPINE SULFATE INJECTION USP should be used with caution in debilitated patients. These patients, especially those with chronic pulmonary disease, may be susceptible to the formation of bronchial mucous plugs due to decreased bronchial secretions.
ATROPINE SULFATE INJECTION USP should be used with caution in elderly patients since they may be more susceptible to its adverse effects. It may cause mental confusion, especially in elderly or brain damaged patients. Elderly patients may react with excitement, agitation, drowsiness or confusion to even small doses of atropine. Changes in dosage should be gradual. Memory may become severely impaired in elderly patients, especially those who already have memory problems, with the continued use of anticholinergics since these drugs block the actions of acetylcholine, which is responsible for many functions of the brain, including memory functions.
Glaucoma :
Conventional parenteral doses of atropine may precipitate acute glaucoma in susceptible individuals.
Myasthenia gravis :
Atropine should be used with extreme caution in patients with myasthenia gravis and should generally only be given to reduce adverse muscarinic effects of an anticholinesterase.
Cardiovascular status :
In conditions characterised by tachycardia, such as cardiac insufficiency or failure, extreme caution must be exercised. Care is also required in cardiac surgery, in patients with acute myocardial infarction or ischaemia as atropine may worsen the symptoms, and in patients with hypertension. Patients with known cardiac problems have developed angina following administration of ATROPINE SULFATE INJECTION USP. ATROPINE SULFATE INJECTION USP has been associated with the development of arrhythmias in adult and paediatric patients. Accelerated heart rate and intraventricular conduction delays have been associated with the develo pment of ventricular fibrillation.
Gastrointestinal :
Since atropine decreases gastrointestinal motility, it should be used with caution in patients with gastric ulcer, oesophageal reflux, known or suspected gastrointestinal infections, e.g. Clostridium difficile associated diarrhoea and colitis (antibiotic associated
pseudomembranous colitis), incomplete intestinal obstruction or ulcerative colitis. ATROPINE SULFATE INJECTION USP should also be used with caution in patients with diarrhoea, since diarrhoea may be an early symptom of incomplete intestinal obstruction,
especially in patients with ileostomy or colostomy.
Down’s syndrome and albinism :
Persons with Down’s syndrome appear to have an increased susceptibility to some of the actions of atropine, whereas those with albinism may have a reduced susceptibility.
Pregnancy : Category A
Although ATROPINE SULFATE INJECTION USP has been taken by a large number of pregnant women and women of child-bearing age without an increase in the frequency of malformations or other direct or indirect harmful effects on the foetus being observed, the safety of atropine in pregnancy has not been positively established. As with all other drugs, caution must be exercised in the use of atropine in pregnant women and women of child-bearing age. ATROPINE SULFATE INJECTION USP crosses the placental barrier and may cause tachycardia in the foetus.
Nursing mothers :
Small amounts of atropine have been found in human breast milk, therefore atropine should only be administered to breast-feeding mothers if absolutely necessary. ATROPINE SULFHATE INJECTION USP may cause antimuscarinic effects in the infant. It may inhibit lactation.
Effect on the ability to drive or operate machinery :
Systemic administration of antimuscarinics may cause drowsiness, blurred vision, dizziness and other effects that may impair a patient’s ability to perform tasks requiring mental alertness and/or visual acuity (such as driving or operating machinery).
MAOIs :
Inhibition of drug metabolising enzymes by MAOIs may possibly enhance the effects of atropine.
Opioid (narcotic) analgesics :
Concurrent use with anticholinergics may result in increased risk of severe constipation, which may lead to paralytic ileus and/or urinary retention.
Urinary Alkalizers :
Urinary excretion of atropine may be delayed by alkalization of the urine, thus potentiating its effects.
Absorption :
The absorption of other drugs may be affected by the reduction in gastric motility caused by atropine.
Ketoconazole :
Anticholinergics may increase gastrointestinal pH, possibly resulting in a marked reduction in ketoconazole absorption during concurrent use. If concomitant therapy is necessary, atropine should be given at least two hours after oral ketoconazole.
Antagonist interactions :
ATROPINE SULFATE INJECTION USP antagonises the actions of a number of compounds, including
• Synthetic choline esters e.g. bethanecol, carbachol
• Anticholinesterase drugs e.g. physostigmine, neostigmine, pyri dostigmine
• Cholinomimetic alkaloids e.g. pilocarpine
• Parasympathomimetics (each may counteract the effect of the other)
Cisapride and metoclopramide :
Concurrent use with anticholinergics may antagonise the effects of cisapride and metoclopramide on gastrointestinal motility.
Haloperidol :
Antipsychotic effectiveness of haloperidol may be decreased in schizophrenic patients.
Cholinesterase inhibitors :
In view of the pharmacodynamic effects of atropine, atropine may interfere with the activity of cholinesterase inhibitors such as rivastigmine, donepezil.
ATROPINE SULFATE INJECTION USP has been reported to be incompatible with alkaline solutions and solutions containing the following : adrenaline hydrochloride, amylobarbitone sodium, ampicillin sodium, chloramphenicol sodium succinate, chlortetracycline
hydrochloride, cimetidine, heparin sodium, hydroxybenzoate preservatives, metaraminol bitartrate, methicillin sodium, methohexitone sodium, nitrofurantoin sodium, novobiocin sodium, oxacillin sodium, pentobarbitone sodium, sodium bicarbonate,sulphadiazine sodium, sulphafurazole diethanolamine, tetracycline hydrochloride, thiopentone sodium, vitamin B complex with ascorbic acid, warfarin sodium. This list is not exhaustive.
SIDE EFFECTS :
Most side effects are directly related to the antimuscarinic actions of ATROPINE SULFATE INJECTION USP. Adverse effects following single or repeated doses are most often the result of excessive dosage.
Cardiovascular :
Transient bradycardia followed by tachycardia with palpitations and arrhythmia. Atropine blocks vagal impulses with consequent increase in heart rate with possible atrial arrhythmias, atrioventricular dissociation, multiple ventricular ectopics and angina. The development of angina in patients with known cardiac problems has been reported. Hypertensive crises and atrioventricular block have also been reported.
Central Nervous System :
Dryness of the mouth with difficulty in swallowing or talking, thirst. These are due to the reduction of salivary, bronchial and sweat secretions and are dose related. Active and passive functions of the eustachian tube may be affected. Tremor, fatigue, drowsiness, ataxia, mental confusion and/or excitement, dizziness, loss of taste, headache, nervousness, weakness, nausea, vomiting, insomnia, psychotic reactions, sedation and seizures. Anhidrosis also may occur and produce heat intolerance in patients living in a hot environment. The inhibition of sweat secretions may also result in hyperthermia.
Gastrointestinal :
Constipation due to inhibition of parasympathetic control of the GI tract. Paralytic ileus. Nausea, vomiting, retrosternal pain due to increased gastric reflux, bloated feeling.
Genitourinary :
Urinary difficulty and retention due to inhibition of parasympathetic control of the bladder.
Ocular :
Visual changes, including blurred vision. Dilatation of the pupils (mydriasis) with loss of accommodation (cycloplegia) and photophobia can occur with increasing doses of atropine. Increased ocular tension.
Dermatological :
Flushing and dryness of the skin. Hypersensitivity reactions may manifest as conjunctivitis or skin rash which, in some instances, progresses to exfoliation and various dermal manifestations.
Other :
More Common : Redness or other signs of irritation at the injection site.
OVERDOSAGE :
Symptoms :
Acute overdosage with atropine produces both peripheral and central signs and symptoms characterised by dilated pupils, difficulty swallowing, hot dry skin, vasodilation and urinary retention. A rash may appear on the face or upper trunk. Tachycardia and hypertension with arrhythmias, anxiety, delirium, hallucinations, hyperactivity, convulsions, marked dryness of the mouth, photophobia, raised body temperature, leucocytosis, nausea, vomiting and restlessness also occur. In severe overdosage, CNS depression, circulatory collapse and hypotension may be followed by coma, skeletal muscle paralysis and death from respiratory and circulatory failure. In addition to tachycardia, cardiac manifestations may include ECG abnormalities (e.g. ventricular arrhythmias, extrasystoles resulting from enhanced re-entrant excitation secondary to reduced conduction velocity. Widening of the QRS complex, prolongation of the QT interval and ST segment depression may also be seen. There is considerable variation in susceptibility to atropine ; recovery has occurred even after 1 g, whereas deaths have been reported from doses of 100 mg or less for adults and 10 mg in children.
TREATMENT OF OVERDOSAGE :
Symptomatic treatment should be instigated to ensure an adequate airway is maintained, fluids are replaced and body temperature is lowered using cold packs and tepid sponging. Artificial respiration with oxygen may be necessary and urinary catheterisation may be required. Hypoxia and acidosis should be corrected and sodium bicarbonate may be given even if acidosis is not present. If photophobia occurs, the patient may be kept in a dark room. Diazepam may be given to control marked excitement and convulsions however the risk of central depression occurring late in the course of atropine poisoning contraindicates large doses of sedative ; phenothiazines should not be given since they may exacerbate antimuscarinic effects. Antiarrhythmics are not recommended if arrhythmias develop. The use of physostigmine as an antidote for atropine poisoning is controversial due to the potential for physostigmine to produce severe adverse effects, e.g. seizures, asystole. The use of physostigmine should be reserved for treatment of patients with extreme delirium or agitation, patients with repetitive seizures, patients with severe sinus tachycardia or supraventricular tachycardia or unresponsive extreme hyperthermia in patients who fail to respond to alternative therapy. Phystostigmine should not be used to treat cardiac conduction defects or ventricular tacharrhythmias. I.V. propranolol may be useful for treatment of supraventricular tacharrhythmias unresponsive to physostigmine or where phystostigmine is contraindicated.Physostigmine should be used with caution in the presence of asthma, gangrene, cardiovascular disease or mechanical obstruction of the gastrointestinal or genitourinary tract. Physostigmine should be used in these circumstances only if a life-threatening emergency occurs. Dialysis is not effective in atropine overdose.
PHARMACEUTICAL PRECAUTIONS :
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
STORAGE :
Store below 30°C (86°F), protected from light.
Do not refrigerate.
SHELF LIFE :
24 months from the date of manufacture.
PRESENTATION :
ATROPINE SULFATE INJECTION USP contains Atropine Sulfate USP 0.5 mg/ml solution.
10 Ampoules of 1 ml in a carton.
Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.

 Cardiovascular
Cardiovascular