1 mg/ml
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
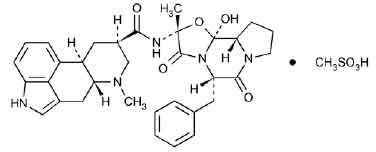
DIHYDROERGOTAMINE MESYLATE INJECTION USP (Dihydroergotamine Mesylate) is an ergot alkaloid used to treat migraines. Dihydroergotamine Mesylate is known chemically as ergotaman-3’, 6’, 18-trione, 9, 10-dihydro-12’-hydroxy-2’-methyl-5’- (phenylmethyl) (5’α)-, monomethanesulfonate (salt). The molecular formula is C33H37N5O5,CH4O3S and molecular weight is 679.78.
STRUCTURAL FORMULA :
Its structural formula is :
DIHYDROERGOTAMINE MESYLATE INJECTION USP is sterile, clear, colourless solution, 1 ml solution filled in 2 ml vial.
COMPOSITION :
Each ml contains :
Dihydroergotamine Mesylate USP 1 mg
Absolute Alcohol USP 6.0 % v/v
Water for Injection USP q.s.
ACTIONS :
Dihydroergotamine binds with high affinity to 5-HT1Dα and 5-HT1Dβ receptors. It also binds with high affinity to serotonin 5-HT1A, 5-HT2A, and 5-HT2C receptors, noradrenaline α2A, α2B and α, receptors, and dopamine D2L and D3 receptors. The therapeutic activity of dihydroergotamine in migraine is generally attributed to the agonist effect at 5-HT1D receptors. Two current theories have been proposed to explain the efficacy of 5-HT1D receptor agonists in migraine. One theory suggests that activation of 5-HT1D receptors located on intracranial blood vessels, including those on arterio-venous anastomoses, leads to vasoconstriction, which correlates with the relief of migraine headache. The alternative hypothesis suggests that activation of 5-HT1D receptors on sensory nerve endings of the trigeminal system results in the inhibition of pro-inflammatory neuropeptide release. In addition, dihydroergotamine possesses oxytocic properties.
PHARMACOKINETICS :
Peak plasma-dihydroergotamine concentrations have been attained within about 1 to 2 hours after oral doses, about 30 minutes after intramuscular injection, about 15 to 45 minutes after subcutaneous injection, and about 45 to 55 minutes after intranasal spray. However, the bioavailability of dihydroergotamine after oral doses is very low; values ranging from less than 0.1 to 1.5% have been reported. Although dihydroergotamine is incompletely absorbed from the gastrointestinal tract, the low bioavailability is considered to be determined primarily by extensive first-pass hepatic metabolism. Its 93 % bound to plasma protein. Dihydroergotamine undergoes extensive metabolism, the major metabolite, 8′-β-hydroxydihydroergotamine, being pharmacologically active. Plasma concentrations of this metabolite are greater than those of dihydroergotamine. A further oxidation step produces 8’ ,10’ -dihydroxydihydroergotamine, which is also pharmacologically active. Other metabolites are also formed. Most of a dose is excreted as metabolites, mainly in the bile; 5 to 10% is excreted in the urine of which only trace amounts are of unchanged drug. The elimination of dihydroergotamine is biphasic; half-lives of about 1 to 2 hours and 22 to 32 hours have been reported for the 2 phases, respectively.
INDICATIONS :
DIHYDROERGOTAMINE MESYLATE INJECTION USP is indicated for the acute treatment of migraine headaches with or without aura and the acute treatment of cluster headache episodes.
Administration :
For Intramuscular, intravenous and subcutaneous use.
Dosage :
For the treatment of migraine and to terminate an acute attack of cluster headache, dihydroergotamine mesylate is usually given by subcutaneous or intramuscular injection in doses of 1 mg repeated, if necessary, after 30 to 60 minutes up to a maximum daily dose of 3 mg. If a more rapid effect is desired it may be given intravenously in doses of 0.5 or 1 mg up to a maximum daily dose of 2 mg. The total weekly dose given by any route of injection should not exceed 6 mg.
CONTRAINDICATIONS :
There have been a few reports of serious adverse events associated with the coadministration of dihydroergotamine and potent CYP 3A4 inhibitors, such as protease inhibitors and macrolide antibiotics, resulting in vasospasm that led to cerebral ischaemia and/or ischaemia of the extremities. The use of potent CYP 3A4 inhibitors (ritonavir, nelfinavir, indinavir, erythromycin, clarithromycin, troleandomycin, ketoconazole, itraconazole) with dihydroergotamine is, therefore contraindicated.
DIHYDROERGOTAMINE MESYLATE INJECTION USP should not be given to patients with ischaemic heart disease (angina pectoris, history of myocardial infarction, or documented silent ischaemia) or to patients who have clinical symptoms or findings consistent with coronary artery vasospasm including Prinzmetal’s variant angina. Because DIHYDROERGOTAMINE MESYLATE INJECTION USP may increase blood pressure, it should not be given to patients with uncontrolled hypertension. DIHYDROERGOTAMINE MESYLATE INJECTION USP, 5-HT1 agonists (e.g., sumatriptan), ergotamine-containing or ergot-type medications or methysergide should not be used within 24 hours of each other.
DIHYDROERGOTAMINE MESYLATE INJECTION USP should not be administered to patients with hemiplegic or basilar migraine. In addition to those conditions mentioned above, DIHYDROERGOTAMINE MESYLATE INJECTION USP is also contraindicated in patients with known peripheral arterial disease, sepsis, following vascular surgery and severely impaired hepatic or renal function. DIHYDROERGOTAMINE MESYLATE INJECTION USP is contraindicated in patients who have previously shown hypersensitivity to ergot alkaloids. DIHYDROERGOTAMINE MESYLATE INJECTION USP should not be used by nursing mothers. Dihydroergotamine mesylate should not be used with peripheral and central vasoconstrictors because the combination may result in additive or synergistic elevation of blood pressure.
WARNINGS :
DIHYDROERGOTAMINE MESYLATE INJECTION USP should only be used where a clear diagnosis of migraine headache has been established.
CYP 3A4 Inhibitors (e.g., Macrolide Antibiotics and Protease Inhibitors) :
There have been rare reports of serious adverse events in connection with the co administration of dihydroergotamine and potent CYP 3A4 inhibitors, such as protease inhibitors and macrolide antibiotics, resulting in vasospasm that led to cerebral ischaemia and/or ischaemia of the extremities. The use of potent CYP 3A4 inhibitors with dihydroergotamine should therefore be avoided Examples of some of the more potent CYP 3A4 inhibitors include : anti-fungals ketoconazole and itraconazole, the protease inhibitors ritonavir, nelfinavir, and indinavir, and macrolide antibiotics erythromycin, clarithromycin, and troleandomycin. Other less potent CYP 3A4 inhibitors should be administered with caution. Less potent inhibitors include saquinavir, nefazodone, fluconazole, grapefruit juice, fluoxetine, fluvoxamine, zileuton, and clotrimazole. These lists are not exhaustive, and the prescriber should consider the effects on CYP 3A4 of other agents being considered for concomitant use with dihydroergotamine.
Fibrotic Complications :
There have been reports of pleural and retroperitoneal fibrosis in patients following prolonged daily use of injectable dihydroergotamine mesylate. Rarely, prolonged daily use of other ergot alkaloid drugs has been associated with cardiac valvular fibrosis. Rare cases have also been reported in association with the use of injectable dihydroergotamine mesylate; however, in those cases, patients also received drugs known to be associated with cardiac valvular fibrosis. Administration of DIHYDROERGOTAMINE MESYLATE INJECTION USP should not exceed the dosing guidelines and should not be used for chronic daily administration.
Risk of Myocardial Ischaemia and/or Infarction and Other Adverse Cardiac Events :
DIHYDROERGOTAMINE MESYLATE INJECTION USP should not be used by patients with documented ischemic or vasospastic coronary artery disease. It is strongly recommended that DIHYDROERGOTAMINE MESYLATE INJECTION USP not be given to patients in whom unrecognized coronary artery disease (CAD) is predicted by the presence of risk factors (e.g., hypertension, hypercholesterolemia, smoker, obesity, diabetes, strong family history of CAD, females who are surgically or physiologically postmenopausal, or males who are over 40 years of age) unless a cardiovascular evaluation provides satisfactory clinical evidence that the patient is reasonably free of coronary artery and ischemic myocardial disease or other significant underlying cardiovascular disease.
The sensitivity of cardiac diagnostic procedures to detect cardiovascular disease or predisposition to coronary artery vasospasm is modest, at best. If, during the cardiovascular evaluation, the patient’s medical history or electrocardiographic investigations reveal findings indicative of or consistent with coronary artery vasospasm or myocardial ischaemia, DIHYDROERGOTAMINE MESYLATE INJECTION USP should not be administered. For patients with risk factors predictive of CAD who are determined to have a satisfactory cardiovascular evaluation, it is strongly recommended that administration of the first dose of DIHYDROERGOTAMINE MESYLATE INJECTION USP take place in the setting of a physician’s office or similar medically staffed and equipped facility unless the patient has previously received dihydroergotamine mesylate. Because cardiac ischaemia can occur in the absence of clinical symptoms, consideration should be given to obtaining on the first occasion of use an electrocardiogram (ECG) during the interval immediately following DIHYDROERGOTAMINE MESYLATE INJECTION USP, in those patients with risk factors. It is recommended that patients who are intermittent long-term users of DIHYDROERGOTAMINE MESYLATE INJECTION USP and who have or acquire risk factors predictive of CAD, as described above, undergo periodic interval cardiovascular evaluation as they continue to use DIHYDROERGOTAMINE MESYLATE INJECTION USP. The systematic approach described above is currently recommended as a method to identify patients in whom DIHYDROERGOTAMINE MESYLATE INJECTION USP may be used to treat migraine headaches with an acceptable margin of cardiovascular safety.
Cardiac Events and Fatalities :
The potential for adverse cardiac events exists. Serious adverse cardiac events, including acute myocardial infarction, life-threatening disturbances of cardiac rhythm, and death have been reported to have occurred following the administration of DIHYDROERGOTAMINE MESYLATE INJECTION USP. Considering the extent of use of dihydroergotamine mesylate in patients with migraine, the incidence of these events is extremely low.
Drug-Associated Cerebrovascular Events and Fatalities :
Cerebral haemorrhage, subarachnoid haemorrhage, stroke, and other cerebrovascular events have been reported in patients treated with DIHYDROERGOTAMINE MESYLATE INJECTION USP; and some have resulted in fatalities. In a number of cases, it appears possible that the cerebrovascular events were primary, the DIHYDROERGOTAMINE MESYLATE INJECTION USP having been administered in the incorrect belief that the symptoms experienced were a consequence of migraine, when they were not. It should be noted that patients with migraine may be at increased risk of certain cerebrovascular events (e.g., stroke, haemorrhage, transient ischaemic attack).
Other Vasospasm Related Events :
DIHYDROERGOTAMINE MESYLATE INJECTION USP, like other ergot alkaloids, may cause vasospastic reactions other than coronary artery vasospasm. Myocardial, peripheral vascular, and colonic ischaemia have been reported with DIHYDROERGOTAMINE MESYLATE INJECTION USP. DIHYDROERGOTAMINE MESYLATE INJECTION USP associated vasospastic phenomena may also cause muscle pains, numbness, coldness, pallor, and cyanosis of the digits. In patients with compromised circulation, persistent vasospasm may result in gangrene or death. DIHYDROERGOTAMINE MESYLATE INJECTION USP should be discontinued immediately if signs or symptoms of vasoconstriction develop.
Increase in Blood Pressure :
Significant elevation in blood pressure has been reported on rare occasions in patients with and without a history of hypertension treated with DIHYDROERGOTAMINE MESYLATE INJECTION USP. DIHYDROERGOTAMINE MESYLATE INJECTION USP is contraindicated in patients with uncontrolled hypertension. An 18 % increase in mean pulmonary artery pressure was seen following dosing with another 5-HT1 agonist in a study evaluating subjects undergoing cardiac catheterization.
PRECAUTIONS :
DIHYDROERGOTAMINE MESYLATE INJECTION USP may cause coronary artery vasospasm; patients who experience signs or symptoms suggestive of angina following its administration should, therefore, be evaluated for the presence of CAD or a predisposition to variant angina before receiving additional doses. Similarly, patients who experience other symptoms or signs suggestive of decreased arterial flow, such as ischemic bowel syndrome or Raynaud’s syndrome following the use of any 5-HT agonist are candidates for further evaluation.
Pregnancy : Category X
DIHYDROERGOTAMINE MESYLATE INJECTION USP may cause foetal harm when administered to a pregnant woman. Dihydroergotamine possesses oxytocic properties and, therefore, should not be administered during pregnancy. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the foetus. There are no adequate studies of dihydroergotamine in human pregnancy, but developmental toxicity has been demonstrated in experimental animals. In embryo-foetal development studies of dihydroergotamine mesylate nasal spray, intranasal administration to pregnant rats throughout the period of organogenesis resulted in decreased foetal body weights and/or skeletal ossification at doses of 0.16 mg/day (associated with maternal plasma dihydroergotamine exposures [AUC] approximately 0.4 to 1.2 times the exposures in humans receiving the MRDD of 4 mg) or greater. A no effect level for embryo-foetal toxicity was not established in rats. Delayed skeletal ossification was also noted in rabbit foetuses following intranasal administration of 3.6 mg/day (maternal exposures approximately 7 times human exposures at the MRDD) during organogenesis. A no effect level was seen at 1.2 mg/day (maternal exposures approximately 2.5 times human exposures at the MRDD). When dihydroergotamine mesylate nasal spray was administered intranasally to female rats during pregnancy and lactation, decreased body weights and impaired reproductive function (decreased mating indices) were observed in the offspring at doses of 0.16 mg/day or greater. A no effect level was not established. Effects on development occurred at doses below those that produced evidence of significant maternal toxicity in these studies. Dihydroergotamine-induced intrauterine growth retardation has been attributed to reduced uteroplacental blood flow resulting from prolonged vasoconstriction of the uterine vessels and/or increased myometrial tone.
Nursing mothers :
Ergot drugs are known to inhibit prolactin. It is likely that DIHYDROERGOTAMINE MESYLATE INJECTION USP is excreted in human milk, but there are no data on the concentration of dihydroergotamine in human milk. It is known that ergotamine is excreted in breast milk and may cause vomiting, diarrhoea, weak pulse, and unstable blood pressure in nursing infants. Because of the potential for these serious adverse events in nursing infants exposed to DIHYDROERGOTAMINE MESYLATE INJECTION USP, nursing should not be undertaken with the use of DIHYDROERGOTAMINE MESYLATE INJECTION USP.
Paediatric Use :
Safety and effectiveness in paediatric patients have not been established.
INTERACTIONS AND INCOMPATIBILITIES :
Vasoconstrictors :
DIHYDROERGOTAMINE MESYLATE INJECTION USP should not be used with peripheral vasoconstrictors because the combination may cause synergistic elevation of blood pressure.
Sumatriptan :
Sumatriptan has been reported to cause coronary artery vasospasm, and its effect could be additive with DIHYDROERGOTAMINE MESYLATE INJECTION USP. Sumatriptan and DIHYDROERGOTAMINE MESYLATE INJECTION USP should not be taken within 24 hours of each other.
Beta Blockers :
Although the results of a clinical study did not indicate a safety problem associated with the administration of DIHYDROERGOTAMINE MESYLATE INJECTION USP to subjects already receiving propranolol, there have been reports that propranolol may potentiate the vasoconstrictive action of ergotamine by blocking the vasodilating property of epinephrine.
Nicotine :
Nicotine may provoke vasoconstriction in some patients, predisposing to a greater ischemic response to ergot therapy.
SSRI’s :
Weakness, hyperreflexia, and incoordination have been reported rarely when 5-HT1 agonists have been co-administered with SSRI’s (e.g., fluoxetine, fluvoxamine, paroxetine, sertraline). There have been no reported cases from spontaneous reports of drug interaction between SSRI’s and DIHYDROERGOTAMINE MESYLATE INJECTION USP.
Oral Contraceptives :
The effect of oral contraceptives on the pharmacokinetics of DIHYDROERGOTAMINE MESYLATE INJECTION USP has not been studied.
ADVERSE EFFECTS :
Serious cardiac events, including some that have been fatal, have occurred following use of DIHYDROERGOTAMINE MESYLATE INJECTION USP, but are extremely rare. Events reported have included coronary artery vasospasm, transient myocardial ischaemia, myocardial infarction, ventricular tachycardia, and ventricular fibrillation. Fibrotic complications have been reported in association with long term use of injectable dihydroergotamine mesylate.
INFORMATION FOR PATIENTS :
Patients should be advised to report to the physician immediately any of the following : numbness or tingling in the fingers and toes, muscle pain in the arms and legs, weakness in the legs, pain in the chest, temporary speeding or slowing of the heart rate, swelling, or itching. Prior to the initial use of the product by a patient, the prescriber should take steps to ensure that the patient understands how to use the product as provided. Administration of DIHYDROERGOTAMINE MESYLATE INJECTION USP should not exceed the dosing guidelines and should not be used for chronic daily administration.
OVERDOSAGE :
To date, there have been no reports of acute overdosage with this drug. Due to the risk of vascular spasm, exceeding the recommended dosages of DIHYDROERGOTAMINE MESYLATE INJECTION USP is to be avoided. Excessive doses of dihydroergotamine may result in peripheral signs and symptoms of ergotism. Treatment includes discontinuance of the drug, local application of warmth to the affected area, the administration of vasodilators, and nursing care to prevent tissue damage. In general, the symptoms of an acute DIHYDROERGOTAMINE MESYLATE INJECTION USP overdose are similar to those of an ergotamine overdose, although there is less pronounced nausea and vomiting with DIHYDROERGOTAMINE MESYLATE INJECTION USP.
The symptoms of an ergotamine overdose include the following :
numbness, tingling, pain, and cyanosis of the extremities associated with diminished or absent peripheral pulses; respiratory depression; an increase and/or decrease in blood pressure, usually in that order; confusion, delirium, convulsions, and coma; and/or some degree of nausea, vomiting, and abdominal pain. In laboratory animals, significant lethality occurs when dihydroergotamine is given at I.V. doses of 44 mg/kg in mice, 130 mg/kg in rats, and 37 mg/kg in rabbits.
TREATMENT OF OVERDOSAGE :
Treatment of acute poisoning with ergotamine is symptomatic. In both acute and chronic poisoning, attempts must be made to maintain an adequate circulation to the affected parts of the body in order to prevent the onset of gangrene. In severe arterial vasospasm vasodilators such as sodium nitroprusside by intravenous infusion have been given; heparin and dextran 40 have also been advocated to minimise the risk of thrombosis. Analgesics may be required for severe ischaemic pain.
PHARMACEUTICAL PRECAUTIONS :
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
STORAGE :
Store below 30°C (86°F), protected from light.
Do not refrigerate.
Avoid excessive heat.
SHELF LIFE :
24 months from the date of manufacture.
PRESENTATION :
DIHYDROERGOTAMINE MESYLATE INJECTION USP is supplied as 1 mg Dihydroergotamine Mesylate in 1 ml aqueous solution.
Such 100 vials are packed in a box.
Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.

 Cardiovascular
Cardiovascular