25 mg + 250 mg
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
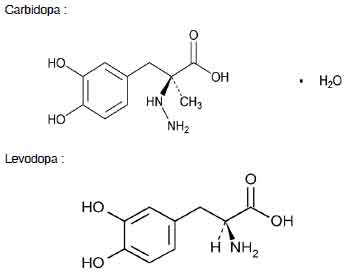
CARBIDOPA AND LEVODOPA TABLETS are a combination of carbidopa and levodopa for the treatment of parkinson’s disease and syndrome. Carbidopa is a peripheral dopa-decarboxylase inhibitor with little or no pharmacological activity when given alone in usual doses. Levodopa a naturally occurring amino acid, is the immediate precursor of the neurotransmitter dopamine. Chemically, carbidopa is benzenepropanoic acid, α -hydrazino-3, 4-dihydroxy-α-methyl-, monohydrate, (s)-; (-)l-α-hydrazino-3, 4-dihydroxy-α-methylhydrocinnamic acid monohydrate. The molecular formula is C10H14N2O4 .H2O and the molecular weight is 244.24. Chemically, levodopa is l-tyrosine, 3-hydroxy-. (-)-3-(3,4-dihydroxyphenyl)-l-alanine. The molecular formula is C9H11NO4 and molecular weight is 197.19.
STRUCTURAL FORMULA :
Its structural formula is :
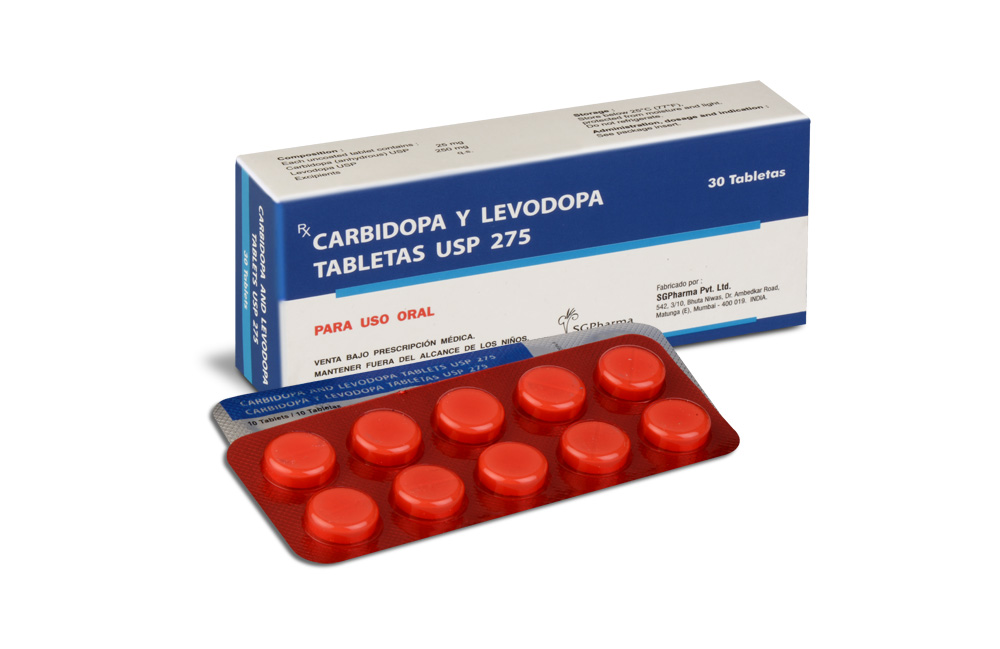
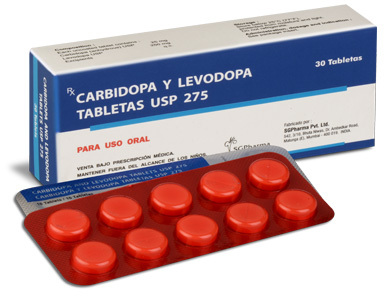
CARBIDOPA AND LEVODOPA TABLETS 275 are white, to off white circular,flat,uncoated tablet with breakline on one side and other side is plain.
ACTIONS :
When levodopa is administered orally it is rapidly decarboxylated to dopamine in extracerebral tissues so that only a small portion of a given dose is transported unchanged to the central nervous system. For this reason, large doses of levodopa are required for adequate therapeutic effect, and these may often be accompanied by nausea and other adverse reactions, some of which are attributable to dopamine formed in extracerebral tissues. Since levodopa competes with certain amino acids for transport across the gut wall, the absorption of levodopa may be impaired in some patients on a high protein diet. Carbidopa inhibits decarboxylation of peripheral levodopa. It does not cross the blood-brain barrier and does not affect the metabolism of levodopa within the central nervous system.The incidence of levodopa-induced nausea and vomiting is less with the combination product than with levodopa. In many patients, this reduction in nausea and vomiting will permit more rapid dosage titration. Since its decarboxylase inhibiting activity is limited to extracerebral tissues, administration of carbidopa with levodopa makes more levodopa available for transport to the brain.
Administration :
CARBIDOPA AND LEVODOPA TABLETS are for oral administration.
Dosage :
The optimum daily dosage of CARBIDOPA AND LEVODOPA TABLETS must be determined by careful titration in each patient. Carbidopa and levodopa tablets are available in a 1:4 ratio of carbidopa to levodopa (25 mg/100 mg) as well as a 1:10 ratio (25 mg/250 mg and 10 mg/100 mg). Tablets of the two ratios may be given separately or combined as needed to provide the optimum dosage. Studies show that peripheral dopa decarboxylase is saturated by carbidopa at approximately 70 to 100 mg a day. Patients receiving less than this amount of carbidopa are more likely to experience nausea and vomiting.
Usual Initial Dosage :
Dosage is best initiated with one CARBIDOPA AND LEVODOPA TABLET 25 mg/100 mg three times a day. This dosage schedule provides 75 mg of carbidopa per day. Dosage may be increased by one tablet every day or every other day, as necessary, until a dosage of eight carbidopa and levodopa tablets 25 mg/100 mg a day is reached. If CARBIDOPA AND LEVODOPA TABLETS, 10 mg/100 mg are used, dosage may be initiated with one tablet three or four times a day. However, this will not provide an adequate amount of carbidopa for many patients. Dosage may be increased by one tablet every day or every other day until a total of eight tablets (2 tablets q.i.d.) is reached.
How to Transfer Patients From Levodopa :
Levodopa must be discontinued at least twelve hours before starting this combination product. A daily dosage of CARBIDOPA AND LEVODOPA TABLETS should be chosen that will provide approximately 25 % of the previous levodopa dosage. Patients who are taking less than 1500 mg of levodopa a day should be started on one carbidopa and levodopa tablet, 25 mg/100 mg three or four times a day. The suggested starting dosage for most patients taking more than 1500 mg of levodopa is one carbidopa and levodopa tablet, 25 mg/250 mg three or four times a day.
Maintenance :
Therapy should be individualized and adjusted according to the desired therapeutic response. At least 70 to 100 mg of carbidopa per day should be provided. When a greater proportion of carbidopa is required, one CARBIDOPA AND LEVODOPA TABLETS 25 mg/100 mg may be substituted for each CARBIDOPA AND LEVODOPA TABLETS 10 mg/100 mg. When more levodopa is required, each CARBIDOPA AND LEVODOPA TABLETS 25 mg/250 mg should be substituted for a CARBIDOPA AND LEVODOPA TABLETS 25 mg/100 mg or a CARBIDOPA AND LEVODOPA TABLETS 10 mg/100 mg. If necessary, the dosage of CARBIDOPA AND LEVODOPA TABLETS 25 mg/250 mg may be increased by one-half or one tablet every day or every other day to a maximum of eight tablets a day. Experience with total daily dosages of carbidopa greater than 200 mg is limited. Because both therapeutic and adverse responses occur more rapidly with this combination product than with levodopa alone, patients should be monitored closely during the dose adjustment period. Specifically, involuntary movements will occur more rapidly with carbidopa and levodopa than with levodopa. The occurrence of involuntary movements may require dosage reduction. Blepharospasm may be a useful early sign of excess dosage in some patients.
Addition of Other Antiparkinsonian Medications :
Standard drugs for Parkinson’s disease, other than levodopa without a decarboxylase inhibitor, may be used concomitantly while carbidopa and levodopa therapy is being administered, although dosage adjustments may be required.
Interruption of Therapy :
Sporadic cases of a symptom complex resembling Neuroleptic Malignant Syndrome (NMS) have been associated with dose reductions and withdrawal of CARBIDOPA AND LEVODOPA TABLETS. Patients should be observed carefully if abrupt reduction or discontinuation of carbidopa and levodopa tablets is required, especially if the patient is receiving neuroleptics. If general anesthesia is required, carbidopa and levodopa therapy may be continued as long as the patient is permitted to take fluids and medication by mouth. If therapy is interrupted temporarily, the patient should be observed for symptoms resembling NMS, and the usual daily dosage may be administered as soon as the patient is able to take oral medication.
CONTRAINDICATIONS :
Nonselective monoamine oxidase (MAO) inhibitors are contraindicated for use with CARBIDOPA AND LEVODOPA TABLETS. These inhibitors must be discontinued at least two weeks prior to initiating therapy with CARBIDOPA AND LEVODOPA TABLETS. CARBIDOPA AND LEVODOPA TABLETS may be administered concomitantly with the manufacturer’s recommended dose of an MAO inhibitor with selectivity for MAO type B (e.g. selegiline HCl). CARBIDOPA AND LEVODOPA TABLETS is contraindicated in patients with known hypersensitivity to any component of this medication, and in patients with narrow angle glaucoma. Since levodopa may activate a malignant melanoma, CARBIDOPA AND LEVODOPA TABLETS should not be used in patients with suspicious undiagnosed skin lesions or a history of melanoma.
WARNINGS :
When this combination product is to be given to patients who are being treated with levodopa, levodopa must be discontinued at least twelve hours before therapy with this product is started. In order to reduce adverse reactions, it is necessary to individualize therapy.
The addition of carbidopa with levodopa in the form of the combination product reduces the peripheral effects (nausea, vomiting) due to decarboxylation of levodopa ; however, carbidopa does not decrease the adverse reactions due to the central effects of levodopa. Because carbidopa permits more levodopa to reach the brain and more dopamine to be formed, certain adverse CNS effects, e.g., dyskinesias (involuntary movements), may occur at lower dosages and sooner with the combination product than with levodopa alone.
Levodopa alone, as well as carbidopa and levodopa, is associated with dyskinesias. The occurrence of dyskinesias may require dosage reduction. As with levodopa, the combination product may cause mental disturbances. These reactions are thought to be due to increased brain dopamine following administration of levodopa. All patients should be observed carefully for the development of depression with concomitant suicidal tendencies. Patients with past or current psychoses should be treated with caution. Carbidopa and levodopa should be administered cautiously to patients with severe cardiovascular or pulmonary disease, bronchial asthma, renal, hepatic or endocrine disease.
As with levodopa, care should be exercised in administering the combination product to patients with a history of myocardial infarction who have residual atrial, nodal, or ventricular arrhythmias. In such patients, cardiac function should be monitored with particular care during the period of initial dosage adjustment, in a facility with provisions for intensive cardiac care. As with levodopa, treatment with the combination product may increase the possibility of upper gastrointestinal haemorrhage in patients with a history of peptic ulcer.
Neuroleptic Malignant Syndrome (NMS)
Sporadic cases of a symptom complex resembling NMS have been reported in association with dose reductions or withdrawal of therapy with carbidopa and levodopa. Therefore, patients should be observed carefully when the dosage of carbidopa and levodopa is reduced abruptly or discontinued, especially if the patient is receiving neuroleptics. NMS is an uncommon but life-threatening syndrome characterized by fever or hyperthermia. Neurological findings, including muscle rigidity, involuntary movements, altered consciousness, mental status changes ; other disturbances, such as autonomic dysfunction, tachycardia, tachypnea, sweating, hyper- or hypotension; laboratory findings, such as creatine phosphokinase elevation, leukocytosis, myoglobinuria, and increased serum myoglobin have been reported.
The early diagnosis of this condition is important for the appropriate management of these patients. Considering NMS as a possible diagnosis and ruling out other acute illnesses (e.g., pneumonia, systemic infection, etc.) is essential. This may be especially complex if the clinical presentation includes both serious medical illness and untreated or inadequately treated extrapyramidal signs and symptoms (EPS). Other important considerations in the differential diagnosis include central anticholinergic toxicity, heat stroke, drug fever, and primary central nervous system (CNS) pathology.
The management of NMS should include : 1) intensive symptomatic treatment and medical monitoring and 2) treatment of any concomitant serious medical problems for which specific treatments are available. Dopamine agonists, such as bromocriptine, and muscle relaxants, such as dantrolene, are often used in the treatment of NMS, however, their effectiveness has not been demonstrated in controlled studies.
PRECAUTIONS :
General :
As with levodopa, periodic evaluations of hepatic, haematopoietic, cardiovascular, and renal function are recommended during extended therapy. Patients with chronic wide-angle glaucoma may be treated cautiously with carbidopa and levodopa provided the intraocular pressure is well-controlled and the patient is monitored carefully for changes in intraocular pressure during therapy.Dopaminergic agents, including levodopa, may be associated with somnolence and very rarely episodes of sudden onset of sleep. In some cases, these episodes may occur without awareness or warning during daily activities. Patients must be informed of this and advised to exercise caution while driving or operating machines while being treated with dopaminergic agents, including levodopa. Patients who have experienced somnolence and/or an episode of sudden sleep onset must refrain from driving or operating machines.
Melanoma :
Epidemiological studies have shown that patients with Parkinson’s disease have a higher risk (2 to approximately 6 fold higher) of developing melanoma than the general population. Whether the increased risk observed was due to Parkinson’s disease or other factors, such as drugs used to treat Parkinson’s disease, is unclear. For the reasons stated above, patients and providers are advised to monitor for melanomas frequently and on a regular basis when using carbidopa and levodopa for any indication. Ideally, periodic skin examinations should be performed by appropriately qualified individuals (e.g., dermatologists).
Laboratory Tests :
Abnormalities in laboratory tests may include elevations of liver function tests such as alkaline phosphatase, SGOT (AST), SGPT (ALT), lactic dehydrogenase, and bilirubin. Abnormalities in blood urea nitrogen and positive Coombs test have also been reported. Commonly, levels of blood urea nitrogen, creatinine, and uric acid are lower during administration of this combination product than with levodopa. Carbidopa and levodopa may cause a false-positive reaction for urinary ketone bodies when a test tape is used for determination of ketonuria. This reaction will not be altered by boiling the urine specimen. False-negative tests may result with the use of glucose-oxidase methods of testing for glucosuria. Cases of falsely diagnosed pheochromocytoma in patients on carbidopa-levodopa therapy have been reported very rarely. Caution should be exercised when interpreting the plasma and urine levels of catecholamines and their metabolites in patients on levodopa or carbidopa-levodopa therapy.
Drug Interactions :
Caution should be exercised when the following drugs are administered concomitantly with carbidopa and levodopa. Symptomatic postural hypotension has occurred when carbidopa and levodopa was added to the treatment of a patient receiving antihypertensive drugs. Therefore, when therapy with carbidopa and levodopa is started, dosage adjustment of the antihypertensive drug may be required. For patients receiving MAO inhibitors (Type A or B),. Concomitant therapy with selegiline and carbidopa-levodopa may be associated with severe orthostatic hypotension not attributable to carbidopa-levodopa alone. There have been rare reports of adverse reactions, including hypertension and dyskinesia, resulting from the concomitant use of tricyclic antidepressants and carbidopa and levodopa.
Dopamine D2 receptor antagonists (e.g., phenothiazines, butyrophenones, risperidone) and isoniazid may reduce the therapeutic effects of levodopa. In addition, the beneficial effects of levodopa in Parkinson’s disease have been reported to be reversed by phenytoin and papaverine. Patients taking these drugs with carbidopa and levodopa should be carefully observed for loss of therapeutic response. Iron salts may reduce the bioavailability of levodopa and carbidopa. The clinical relevance is unclear. Although metoclopramide may increase the bioavailability of levodopa by increasing gastric emptying, metoclopramide may also adversely affect disease control by its dopamine receptor antagonistic properties. Concomitant therapy with selegiline and carbidopa-levodopa may be associated with severe orthostatic hypotension not attributable to carbidopa-levodopa alone. Since levodopa competes with certain amino acids, the absorption of CARBIDOPA AND LEVODOPA TABLETS may be impaired in some patients on a high protein diet.
The effect of simultaneous administration of antacids with CARBIDOPA AND LEVODOPA TABLETS on the bioavailability of levodopa has not been studied. CARBIDOPA AND LEVODOPA TABLETS may be given to patients with Parkinson’s disease and syndrome who are taking vitamin preparations that contain pyridoxine hydrochloride (Vitamin B 6)
Carcinogenesis, Mutagenesis, Impairment of Fertility :
In a two-year bioassay of carbidopa and levodopa, no evidence of carcinogenicity was found in rats receiving doses of approximately two times the maximum daily human dose of carbidopa and four times the maximum daily human dose of levodopa.
Pregnancy : Pregnancy Category C.
Treatment :

 Cardiovascular
Cardiovascular