100 mg
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
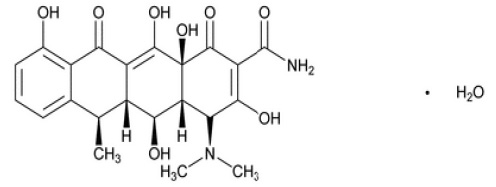
DOXYCYCLINE TABLETS USP (Doxycycline Monohydrate) is a broad-spectrum antibiotic synthetically derived from oxytetracycline or metacycline. Chemically, Doxycycline Monohydrate is 4-(Dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,5,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-2-naphthacenecarboxamide monohydrate. The molecular formula is C22H24N2O8·H2O and molecular weight is 462.45.
STRUCTURAL FORMULA :
Its structural formula is :
DOXYCYCLINE TABLETS USP are greenish yellow coloured, circular, biconvex uncoated tablets with breakline on one side and plain on other side.
COMPOSITION :
Each uncoated tablet contains :
Doxycycline Monohydrate USP
equivalent to Doxycycline (anhydrous) 100 mg
Excipients q.s.
ACTIONS :
Doxycycline is primarily a bacteriostatic antibiotic. The main mechanism of action of doxycycline is on protein synthesis. Doxycycline passes directly through the lipid bilayer of the bacterial cell wall and an energy dependent active transport system pumps the drug through the inner cytoplasmic membrane. Once inside the cell doxycycline inhibits protein synthesis by binding to 30S ribosomes and prevents the addition of amino acids to the growing peptide chain. Doxycycline will impair protein synthesis in mammalian cells at very
high concentrations but these cells lack the active transport system found in bacteria.
MICROBIOLOGY :
The tetracyclines are primarily bacteriostatic and are thought to exert their antimicrobial effect by the inhibition of protein synthesis. The tetracyclines, including doxycycline, have a similar antimicrobial spectrum of activity against a wide range of gram-positive and gram-negative microorganisms. Cross-resistance of these microorganisms to tetracyclines is common. Doxycycline has been shown to be active against most strains of the following microorganisms, both in vitro and in clinical infections as described in the INDICATIONS section.
Aerobic Gram-Positive Microorganisms :
Because many strains of the following groups of gram-positive microorganisms have been shown to be resistant to tetracyclines, culture and susceptibility testing are recommended :
Bacillus anthracis
Listeria monocytogenes
Staphylococcus aureus*
*Doxycycline is not the drug of choice in the treatment of any type of staphylococcal infection. Up to 44 percent of strains of Streptococcus pyogenes and 74 percent of Streptococcus faecalis have been found to be resistant to tetracycline drugs. Therefore, tetracyclines should not be used to treat streptococcal infections unless the microorganism has been demonstrated to be susceptible. Streptococcus pneumoniae
Aerobic Gram-Negative Microorganisms :
Bartonella bacilliformis
Brucella species
Calymmatobacterium granulomatis
Campylobacter foetus
Francisella tularensis
Haemophilus ducreyi
Haemophilus influenzae
Neisseria gonorrhoeae
Vibrio cholerae
Yesinia pestis Because many strains of the following groups of gram-negative microorganisms have been shown to be resistant to tetracyclines, culture and susceptibility testing are recommended :
Acinetobacter species
Enterobacter aerogenes
Escherichia coli
Klebsiella species
Shigella species
Susceptibility Tests :
Dilution techniques :
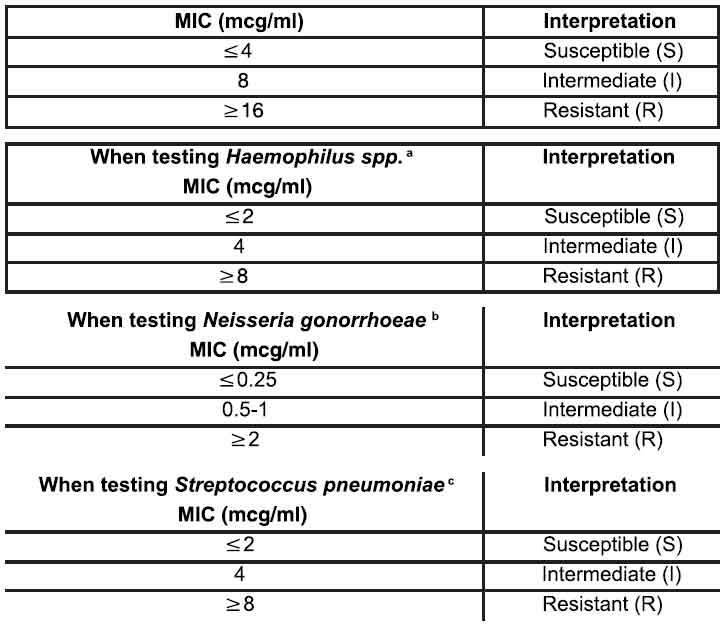
Quantitative methods are used to determine antimicrobial minimum inhibitory concentrations (MICs). These MICs provide estimates of the susceptibility of bacteria to antimicrobial compounds. The MICs should be determined using a standardized procedure. Standardized procedures are based on a dilution method (broth or agar) or equivalent with standardized inoculum concentrations and standardized concentrations of tetracycline powder. The MIC values should be interpreted according to the following criteria for indicated aerobic microorganisms other than Haemophilus species, Neisseria gonorrhoeae, and Streptococcus pneumoniae :
a. Interpretative criteria applicable only to tests performed by broth microdilution method using Haemophilus Test Medium (HTM).
b. Interpretative criteria applicable only to tests performed by agar dilution method using GC agar base with 1 % defined growth supplement.
c. Interpretative criteria applicable only to tests performed by broth microdilution method using cation-adjusted Mueller-Hinton broth with 2 to 5 % lysed horse blood.
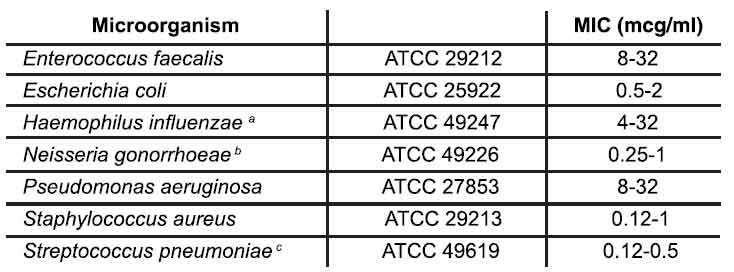
A report of “Susceptible” indicates that the pathogen is likely to be inhibited if the antimicrobial compound in the blood reaches the concentrations usually achievable. A report of “Intermediate” indicates that the result should be considered equivocal, and, if the microorganism is not fully susceptible to alternative, clinically feasible drugs, the test should be repeated. This category implies possible clinical applicability in body sites where the drug is physiologically concentrated or in situations where high dosage of drug can be used. This category also provides a buffer zone, which prevents small uncontrolled technical factors from causing major discrepancies in interpretation. A report of “Resistant” indicates that the pathogen is not likely to be inhibited if the antimicrobial compound in the blood reaches the concentrations usually achievable; other therapy should be selected. Standardized susceptibility test procedures require the use of laboratory control microorganisms to control the technical aspects of the laboratory procedures. Standard tetracycline powder should provide the following MIC values :
a. Range applicable only to tests performed by broth microdilution method using Haemophilus Test Medium (HTM).
b. Range applicable only to tests performed by agar dilution method using GC agar base with 1 % defined growth supplement.
c. Range applicable only to tests performed by broth microdilution method using cation-adjusted Mueller-Hinton broth with 2 to 5 % lysed horse blood.
Diffusion techniques :
Quantitative methods that require measurement of zone diameters also provide reproducible estimates of the susceptibility of bacteria to antimicrobial compounds. One such standardized procedure requires the use of standardized inoculum concentrations. This procedure uses paper disks impregnated with 30-mcg tetracycline or 30-mcg doxycycline to test the susceptibility of microorganisms to doxycycline. Reports from the laboratory providing results of the standard single-disk susceptibility test with 30-mcg tetracycline-class disk or the 30-mcg doxycycline disk should be interpreted according to the following criteria for indicated aerobic microorganisms other than Haemophilus species, Neisseria gonorrhoeae, and Streptococcus pneumoniae :
a. Interpretative criteria applicable only to tests performed by disk diffusion method using a 30-mcg tetracycline class disk and using Haemophilus Test Medium (HTM).
b. Interpretative criteria applicable only to tests performed by disk diffusion method using a 30-mcg tetracycline class disk and using GC agar base with 1 % defined growth supplement.
c. Interpretative criteria applicable only to tests performed by disk diffusion method using a 30-mcg tetracycline class disk and using Mueller-Hinton agar with 5 % defibrinated sheep blood and incubated in 5 % CO2.
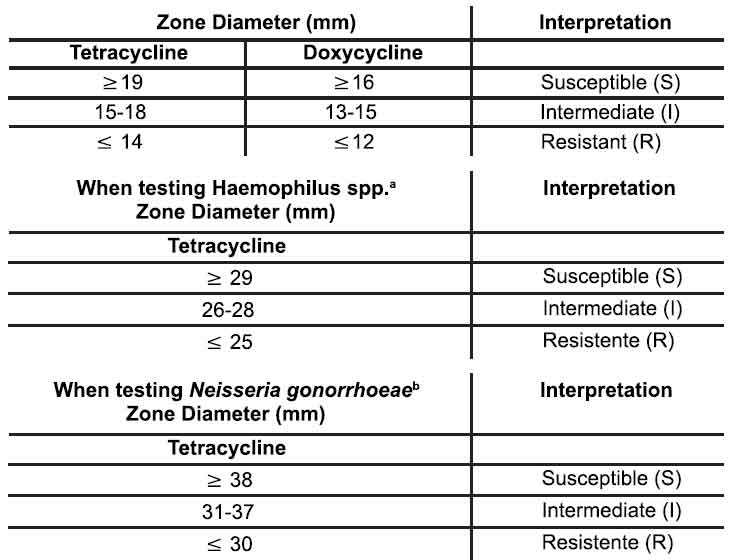
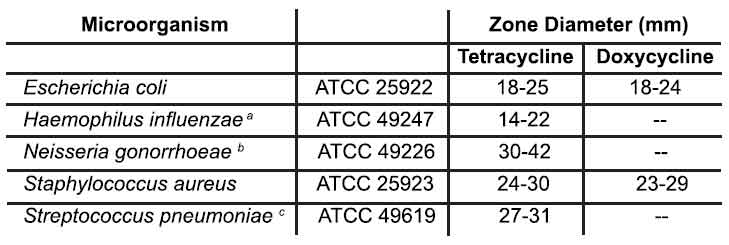
Interpretation should be as stated above for results using dilution techniques. Interpretation involves correlation of the diameter obtained in the disk test with the MIC for tetracycline or doxycycline, respectively. As with standardized dilution techniques, diffusion methods require the use of laboratory control microorganisms that are used to control the technical aspects of the laboratory procedures. For the diffusion technique, the 30-mcg tetracycline-class disk or the 30-mcg doxycycline disk should provide the following zone
diameters in these laboratory test quality control strains :
a. Range applicable only to tests performed by disk diffusion method using a 30-mcg tetracycline-class disk and using Haemophilus Test Medium (HTM).
b. Range applicable only to tests performed by disk diffusion method using a 30-mcg tetracycline-class disk and using GC agar base with 1 % defined growth supplement.
c. Range applicable only to tests performed by disk diffusion method using a 30-mcg tetracycline-class disk and using Mueller-Hinton agar with 5 % defibrinated sheep blood and incubated 5 % CO2.
PHARMACOKINETICS :
Doxycycline is readily and almost completely absorbed from the gastrointestinal tract and absorption is not significantly affected by the presence of milk or food in the stomach or duodenum. Mean peak plasma concentrations of 2.6 micrograms/ml have been reported 2 hours after a 200-mg oral dose, falling to 1.45 micrograms/ml at 24 hours. After intravenous infusion of the same dose peak plasma concentrations are briefly somewhat higher, but become very similar to those after oral dosage on equilibration into the tissues. About 80 to 95 % of doxycycline in the circulation is reported to be bound to plasma proteins. Its biological half-life varies from about 12 to 24 hours. Doxycycline is more lipid-soluble than tetracycline. It is widely distributed in body tissues and fluids. In patients with normal renal function about 40 % of a dose is slowly excreted in the urine, although more is excreted by this route if the urine is made alkaline. However, the majority of a dose of doxycycline is excreted in the faeces after chelation in the intestines. Although doxycycline has been reported to undergo partial inactivation in the liver, some sources consider this doubtful; however, the kinetics of doxycycline have been reportedly altered in patients receiving drugs that induce hepatic metabolism. Doxycycline is stated not to accumulate significantly in patients with renal impairment, although excretion in the urine is reduced; increased amounts of doxycycline are excreted in the faeces in these patients. Nevertheless, there have been reports of some accumulation in renal failure. Removal of doxycycline by haemodialysis is insignificant.
INDICATIONS :
To reduce the development of drug-resistant bacteria and maintain the effectiveness of DOXYCYCLINE TABLETS USP and other antibacterial drugs, DOXYCYCLINE TABLETS USP should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
Doxycycline is indicated for the treatment of the following infections :
Rocky mountain spotted fever, typhus fever and the typhus group, Q fever, rickettsialpox, and tick fevers caused by Rickettsiae.
Respiratory tract infections caused by Mycoplasmapneumoniae.
Lymphogranuloma venereum caused by Chlamydiatrachomatis.
Psittacosis (omithosis) caused by Chlamydiapsittaci.
Trachoma caused by Chlamydiatrachomatis, although the infectious agent is not always eliminated as judged by immunofluorescence.
Inclusion conjunctivitis caused by Chlamydiatrachomatis.
Uncomplicated urethral, endocervical or rectal infections in adults caused by Chlamydiatrachomatis.
Nongonococcal urethritis caused by Ureaplasmaurealyticum.
Relapsing fever due to Borreliarecurrentis.
Administration :
FOR ORAL USE.
Dosage :
THE USUAL DOSAGE AND FREQUENCY OF ADMINISTRATION OF DOXYCYCLINE DIFFERS FROM THAT OF THE OTHER TETRACYCLINES. EXCEEDING THE RECOMMENDED DOSAGE MAY RESULT IN AN INCREASED INCIDENCE OF SIDE EFFECTS.
Adults :
The usual dose of oral doxycycline is 200 mg on the first day of treatment (administered 100 mg every 12 hours or 50 mg every 6 hours) followed by a maintenance dose of 100 mg/day. The maintenance dose may be administered as a single dose or as 50 mg every 12 hours. In the management of more severe infections (particularly chronic infections of the urinary tract), 100 mg every 12 hours is recommended. For paediatric patients above eight years of age : The recommended dosage schedule for paediatric patients weighing 100 pounds or less is 2 mg/lb of body weight divided into two doses on the first day of treatment, followed by 1 mg/lb of body weight given as a single daily dose or divided into two doses, on subsequent days. For more severe infections up to 2 mg/lb of body weight may be used. For paediatric patients over 100 pounds the usual adult dose should be used.
Uncomplicated gonococcal infections in adults (except anorectal infections in men) : 100 mg, by mouth, twice a day for 7 days. As an alternate single visit dose, administer 300 mg stat followed in one hour by a second 300 mg dose.
Acute epididymo-orchitis caused by N. gonorrhoeae : 100 mg, by mouth, twice a day for at least 10 days.
Primary and secondary syphilis : 300 mg a day in divided doses for at least 10 days. Uncomplicated urethral, endocervical, or rectal infection in adults caused
by Chlamydiatrachomatis : 100 mg, by mouth, twice a day for at least 7 days.
Nongonococcal urethritis caused by C. trachomatis and U. urealyticum : 100 mg, by mouth, twice a day for at least 7 days.
Acute epididymo-orchitis caused by C. urachomatis : 100 mg, by mouth, twice a day for at least 10 days.
Inhalational anthrax (post-exposure) :
ADULTS : 100 mg of doxycycline, by mouth, twice a day for 60 days.
CHILDREN : weighing less than 100 pounds (45 kg) : 1 mg/lb (2.2 mg/kg) of body weight, by mouth, twice a day for 60 days. Children weighing 100 pounds or more should receive the adult dose. When used in streptococcal infections, therapy should be continued for 10 days.
Administration of adequate amounts of fluid along with capsule and tablet forms of drugs in the tetracycline class is recommended to wash down the drugs and reduce the risk of oesophageal irritation and ulceration. If gastric irritation occurs, doxycycline may be given with food. Ingestion of a high fat meal has been shown to delay the time to peak plasma concentrations by an average of one hour and 20 minutes. However, in the same study, food enhanced the average peak concentration by 7.5 % and the area under the curve by 5.7 %.
CONTRAINDICATIONS :
This drug is contraindicated in persons who have shown hypersensitivity to any of the tetracyclines. DOXYCYCLINE TABLETS USP contains lactose which is contra-indicated in patients with galactosaemia, the glucose-galactose malabsorption syndrome, or lactase deficiency.
WARNINGS :
THE USE OF DRUGS OF THE TETRACYCLINE CLASS DURING TOOTH DEVELOPMENT (LAST HALF OF PREGNANCY, INFANCY, AND CHILDHOOD TO THE AGE OF 8 YEARS) MAY CAUSE PERMANENT DISCOLORATION OF THE TEETH (YELLOW-GRAY-BROWN). Pseudomembranous colitis has been reported with nearly all antibacterial agents, including doxycycline, and may range in severity from mild to life-threatening. Therefore, it is important to consider this diagnosis in patients who present with diarrhoea subsequent to the administration of antibacterial agents. Treatment with antibacterial agents alters the normal flora of the colon and may permit overgrowth of clostridia. Studies indicate that a toxin produced by Clostridium difficile is a primary cause of “antibiotic-associated colitis.”After the diagnosis of Pseudomembranous colitis has been established, therapeutic measures should be initiated. Mild cases of Pseudomembranous colitis usually respond to discontinuation of the drug alone. In moderate to severe cases, consideration should be given to management with fluids and electrolytes, protein supplementation, and treatment with an antibacterial drug clinically effective against Clostridiumdifficile colitis.
This adverse reaction is more common during long-term use of the drugs but has been observed following repeated short-term courses. Enamel hypoplasia has also been reported. TETRACYCLINE DRUGS, THEREFORE, SHOULD NOT BE USED IN THIS AGE GROUP, EXCEPT FOR ANTHRAX, INCLUDING INHALATIONAL ANTHRAX (POST-EXPOSURE), UNLESS OTHER DRUGS ARE NOT LIKELY TO BE EFFECTIVE OR ARE CONTRAINDICATED. All tetracyclines form a stable calcium complex in any bone-forming tissue. A decrease in the fibula growth rate has been observed in prematures given oral tetracycline in doses of 25 mg/kg every six hours. This reaction was shown to be reversible when the drug was discontinued. Results of animal studies indicate that tetracyclines cross the placenta, are found in fatal tissues, and can have toxic effects on the developing foetus (often related to retardation of skeletal development). Evidence of embryo toxicity has been noted in animals treated early in pregnancy. If any tetracycline is used during pregnancy r if the patient becomes pregnant while taking these drugs, the patient should be apprised of the potential hazard to the foetus. The antianabolic action of the tetracyclines may cause an increase in BUN. Studies to date indicate that this does not occur with the use of doxycycline in patients with impaired renal function.
PRECAUTIONS :
Prescribing DOXYCYCLINE TABLETS USP in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria. As with other antibiotic preparations, use of this drug may result in overgrowth of non-susceptible organisms, including fungi. If super infection occurs, the antibiotic should be discontinued and appropriate therapy instituted. Bulging fontanels in infants and benign intracranial hypertension in adults have been reported in individuals receiving tetracyclines. These conditions disappeared when the drug was discontinued. Incision and drainage or other surgical procedures should be performed in conjunction with antibiotic therapy when indicated. DOXYCYCLINE TABLETS USP should be used cautiously in diabetic patients.
Laboratory Tests :
In venereal disease when coexistent syphilis is suspected, a dark-field examination should be done before treatment is started and the blood serology repeated monthly for at least four months. Photosensitivity manifested by an exaggerated sunburn reaction has been observed in some individuals taking tetracyclines. Patients apt to be exposed to direct sunlight or ultraviolet light should be advised that this reaction can occur with tetracycline drugs, and treatment should be discontinued at the first evidence of skin erythema.
Pregnancy : Category D
Doxycycline is contra-indicated in pregnancy. It appears that the risks associated with the use of tetracyclines during pregnancy are predominantly due to effects on teeth and skeletal development.
Nursing mothers :
Tetracylines are excreted into milk and are therefore contra-indicated in nursing mothers.
Paediatric Use :
In infants and children up to 8 years of age, tetracyclines may cause permanent discoloration of teeth and enamel hypoplasia. Use of doxycycline periodontal system is not recommended. In children 8 years of age and over, safety and efficacy of doxycycline periodontal system have not been established.
EFFECTS ON ABILITY TO DRIVE AND USE MACHINES :
The effect of doxycycline on the ability to drive or operate heavy machinery has not been studied. There is no evidence to suggest that doxycycline may affect these abilities.
INTERACTIONS :
Because tetracyclines have been shown to depress plasma prothrombin activity, patients who are on anticoagulant therapy may require downward adjustment of their anticoagulant dosage. Since bacteriostatic drugs may interfere with the bactericidal action of penicillin, it is advisable to avoid giving tetracyclines in conjunction with penicillin. Absorption of tetracyclines is impaired by antacids containing aluminum, calcium, or magnesium, and iron-containing preparations.
Laboratory Test Interactions :
False elevations of urinary catecholamine levels may occur due to interference with the fluorescence test.
SIDE EFFECTS :
Doxycycline is generally well tolerated.
Due to doxycycline’s virtually complete absorption, side effects of the lower bowel, particularly diarrhoea, have been infrequent. The following adverse reactions have been observed in patients receiving doxycycline.
More common reactions
Dermatological : Photosensitive skin reactions, erythematous rash, maculopapular rash, morbilliform rash, pustular rash, urticaria, photo-onycholysis and discolouration of the nails.
Gastrointestinal : Nausea, anorexia, vomiting, dysphasia, diarrhoea, oesophagitis, oesophageal ulceration, abdominal pain, glossitis, black hairy tongue.
Hypersensitivity reactions : Urticaria, exacerbation of systemic lupus erythematosus.
Hepatic : Cholestatic hepatitis, fatty liver degeneration.
Renal : Dose related increase in serum urea.
Musculoskeletal : Tooth discolouration, enamel hypoplasia.
Others : Bulging fontanelles have been reported in young infants following full
therapeutic dosage. The sign disappeared rapidly when the drug was discontinued. When given over prolonged periods, tetracyclines have been reported to produce brown-black microscopic discolouration of thyroid glands. No abnormalities of thyroid function studies are known to occur.
Less common reactions
Gastrointestinal : Enterocolitis, inflammatory lesions (with monilial overgrowth) in the anogenital region; dyspepsia and Pseudomembranous colitis; C. difficile diarrhoea.
Hepatic : Abnormal hepatic function has been reported rarely (< 1 in 1000), hepatotoxicity.
Skin : Exfoliative dermatitis; Stevens-Johnson syndrome, Toxic Epidermal Necrolysis (TEN).
Musculoskeletal : Arthralgia; myalgia.
INFORMATION FOR PATIENTS :
Patients should be counselled that antibacterial drugs, including DOXYCYCLINE TABLETS USP should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When DOXYCYCLINE TABLETS USP are prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by DOXYCYCLINE TABLETS USP or other antibacterial drugs in the future.
OVERDOSAGE :
Tetracyclines, including doxycycline, generally have low toxicity. Severe toxicity following acute overdosage is unlikely, with nausea and vomiting being the most common effects after ingestion of therapeutic and overdose amounts.
TREATMENT OF OVERDOSAGE :
Treatment may include immediate discontinuation of medication, dilution with water or milk and general supportive care. Antacids may be useful in managing gastric irritation. In most cases, gastrointestinal decontamination is not required. Plasma levels are not clinically useful and specific laboratory monitoring is not needed unless otherwise indicated.
STORAGE :
Store below 25°C (77°F), protected from moisture and light.
Do not refrigerate.
SHELF LIFE :
24 months from the date of manufacture.
PRESENTATION :
DOXYCYCLINE TABLETS USP contains Doxycycline Monohydrate USP equivalent to Doxycycline (anhydrous) 100 mg.
3 Strips of 10 Tablets per Box.
Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.

 Cardiovascular
Cardiovascular