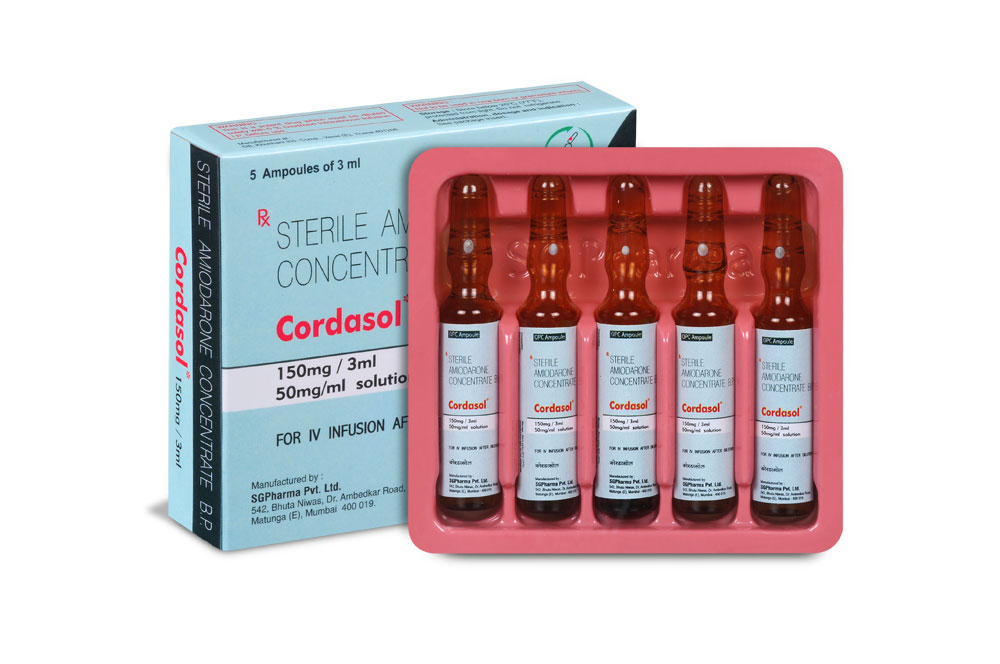
150 mg/3 ml
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
CORDASOL (Amiodarone Hydrochloride) is a class III antiarrhythmic agent. Chemically, Amiodarone Hydrochloride is (2-Butylbenzofuran-3-yl) [4-[2-(diethylamino)ethoxy]-3, 5-diiodophenyl]methanone hydrochloride. Its molecular formula is C25H30ClI2NO3 and its molecular weight is 682.0
-Structure.jpg)
CORDASOL is a sterile, clear, pale yellow, micellar solution filled in 3 ml amber glass ampoules.
COMPOSITION :
Each ml contains :
Amiodarone HCl B.P. 50 mg
Benzyl Alcohol I.P. 2 % v/v
Water for Injections I.P. q.s.
ACTIONS :
Amiodarone is generally considered a class III antiarrhythmic drug, but it possesses electrophysiologic characteristics of all four Vaughan Williams classes. Like class I drugs, amiodarone blocks sodium channels at rapid pacing frequencies, and like class II drugs, it exerts a noncompetitive antisympathetic action, one of its main effects, with prolonged administration, is to lengthen the cardiac action potential, a class III effect. The negative chronotropic effect of amiodarone in nodal tissues is similar to the effect of class IV drugs. In addition to blocking sodium channels, amiodarone blocks myocardial potassium channels, which contributes to slowing of conduction and prolongation of refractoriness. The antisympathetic action and the block of calcium and potassium channels are responsible for the negative dromotropic effects on the sinus node and for the slowing of conduction and prolongation of refractoriness in the atrioventricular (AV) node. Its vasodilatory action can decrease cardiac workload and consequently myocardial oxygen consumption.
Amiodarone I.V. administration prolongs intranodal conduction (Atrial-His, AH) and refractoriness of the atrioventricular node (ERP AVN), but has little or no effect on sinus cycle length (SCL), refractoriness of the right atrium and right ventricle (ERP RA and ERP RV),
repolarization (QTc), intraventricular conduction (QRS), and infranodal conduction (His-ventricular, HV). A comparison of the electrophysiologic effects of Amiodarone I.V. and oral Amiodarone is shown in the table below.
PHARMACOKINETICS :
Amiodarone exhibits complex disposition characteristics after intravenous administration. Peak serum concentrations after single 5 mg/kg 15-minute intravenous infusions in healthy subjects range between 5 and 41 mg/L. Peak concentrations after 10-minute infusions of
150 mg Amiodarone I.V. in patients with ventricular fibrillation (VF) or haemodynamically unstable ventricular tachycardia (VT) range between 7 and 26 mg/L. Due to rapid distribution, serum concentrations decline to 10 % of peak values within 30 to 45 minutes after the end of the infusion. In clinical trials, after 48 hours of continued infusions (125, 500, or 1000 mg/day) plus supplemental (150 mg) infusions (for recurrent arrhythmias), Amiodarone mean serum concentrations between 0.7 to 1.4 mg/L were observed (n = 260). N-
esethylamiodarone (DEA) is the major active metabolite of Amiodarone in humans. DEA serum concentrations above 0.05 mg/L are not usually seen until after several days of continuous infusion but with prolonged therapy reach approximately the same concentration as Amiodarone. The enzymes responsible for the N-deethylation are believed to be the cytochrome P450 3A (CYP3A) subfamily, principally CYP3A4. This isozyme is present in both the liver and intestines.The highly variable systemic availability of oral Amiodarone may be attributed potentially to large interindividual variability in CYP3A4 activity.
Amiodarone is eliminated primarily by hepatic metabolism and biliary excretion and there is negligible excretion of Amiodarone or DEA in urine. Neither Amiodarone nor DEA is dialyzable. Amiodarone and DEA cross the placenta and both appear in breast milk. No data are available on the activity of DEA in humans, but in animals, it has significant electrophysiologic and antiarrhythmic effects generally similar to amiodarone itself. DEA’s precise role and contribution to the antiarrhythmic activity of oral Amiodarone are not certain. The development of maximal ventricular class III effects after oral Amiodarone administration in humans correlates more closely with DEA accumulation over time than with Amiodarone accumulation. On the other hand after Amiodarone I.V. administration, there is evidence of
activity well before significant concentrations of DEA are attained. The following table summarizes the mean ranges of pharmacokinetic parameters of Amiodarone reported in single dose I.V. (5 mg/kg over 15 min) studies of healthy subjects.
Desethylamiodarone clearance and volume involve an unknown biotransformation factor. The systemic availability of oral Amiodarone in healthy subjects ranges between 33 % and 65 %. From in vitro studies, the protein binding of Amiodarone is > 96 %. In clinical studies of 2 to 7 days, clearance of Amiodarone after intravenous administration in patients with VT and VF ranged between 220 and 440 mL/h/kg. Age, sex, renal disease and hepatic disease (cirrhosis) do not have marked effects on the disposition of Amiodarone or DEA. Renal impairment does not influence the pharmacokinetics of amiodarone. After a single dose of Amiodarone I.V. in cirrhotic patients, significantly lower Cmax and average concentration values are seen for DEA, but mean Amiodarone levels are unchanged. Normal subjects over 65 years of age show lower clearances (about 100 mL/hr/kg) than younger subjects (about 150 mL/hr/kg) and an increase in t1/2 from about 20 to 47 days. In patients with severe left ventricular dysfunction, the pharmacokinetics of Amiodarone are not significantly altered but the terminal disposition t1/2 of DEA is prolonged. Although no dosage adjustment for patients with renal, hepatic, or cardiac abnormalities has been defined during chronic treatment with oral Amiodarone, close clinical monitoring is prudent for elderly patients and those with severe left ventricular dysfunction. There is no established relationship between drug concentration and therapeutic response for short-term intravenous use. Steady-state Amiodarone concentrations of 1 to 2.5 mg/L have been associated with antiarrhythmic effects and acceptable toxicity following chronic oral amiodarone therapy.
PHARMACODYNAMICS :
Amiodarone I.V. has been reported to produce negative inotropic and vasodilatory effects in animals and humans. In clinical studies of patients with refractory VF or haemodynamically unstable VT, treatment-emergent, drug-related hypotension occurred in 288 of 1836 patients (16 %) treated with Amiodarone I.V. No correlations were seen between the baseline ejection fraction and the occurrence of clinically significant hypotension during infusion of Amiodarone I.V.
INDICATIONS :
Tachyarrhythmias associated with Wolff-Parkinson-White Syndrome. All types of tachyarrhythmias including; supraventricular, nodal and ventricular tachycardias, atrial flutter and fibrillation; ventricular fibrillation; when other agents cannot be used. The injection is to be used when a rapid response is required. CORDASOL is indicated for initiation of treatment and prophylaxis of frequently recurring ventricular fibrillation and haemodynamically unstable ventricular tachycardia in patients refractory to other therapy. CORDASOL also can be used to treat patients with VT/VF for whom oral Amiodarone is indicated, but who are unable to take oral medication. During or after treatment with CORDASOL, patients may be transferred to oral amiodarone therapy. CORDASOL should be used for acute treatment until the patient’s ventricular arrhythmias are stabilized. Most patients will require this therapy for 48 to 96 hours, but CORDASOL may be safely administered for longer periods if necessary.
Administration :
CORDASOL should only be used when facilities exist for cardiac monitoring and defibrillation, should the need arise. Intravenous injection is generally not advised because of haemodynamic risks (severe hypotension, circulatory collapse). Intravenous infusion should be preferred whenever it is possible.
INSTRUCTION FOR USE OF AMPOULE :
The ampoule used in this product is equipped with O.P.C (One Point Cut) opening system. No ampoule file is needed to open the ampoule. The neck of the ampoule is prescored at the point of constriction. A coloured dot on the ampoule head helps to orientate the ampoule. Take the ampoule and face the coloured dot. Let the solution at the head of the ampoule to flow down by shaking or a gentle stroke. The ampoule opens easily by placing the thumb on the coloured dot and gently pressing downwards as shown.

Dosage :
The standard recommended dose is 5 mg/kg body weight given by intravenous infusion over a period of 20 minutes to 2 hours. This should be administered as a dilute solution in 250 mL 5 % Glucose. CORDASOL is incompatible with saline and should be administered solely in 5 % Glucose solution. This may be followed by repeat infusions up to 1200 mg (approximately 15 mg/kg body weight) in up to 500 mL 5 % glucose per 24 hours, the rate of infusion being adjusted on the basis of clinical response. In extreme clinical emergency CORDASOL may, at the discretion of the clinician, be given as a slow injection of 150 - 300 mg in 10 - 20 mL 5 % Glucose over a minimum of 3 minutes. This should not be repeated for at least 15 minutes. Patients treated in this way must be closely monitored, e.g. in an intensive care unit. Do not mix Amiodarone with other preparations in the same syringe or infusion solution. Solutions containing less than 2 ampoules CORDASOL in 500 mL 5 % Glucose are unstable and should not be used. When given by infusion CORDASOL may reduce drop size and if appropriate, adjustments should be made to the rate of infusion.
Oral therapy should be initiated concomitantly at the usual loading dose i.e. 200 mg 3 times a day as soon as possible after an adequate response has been obtained using CORDASOL which should then be phased out gradually and an overlap of oral and intravenous medication of up to two days is recommended to prevent plasma levels falling and efficacy being lost. Repeated or continuous infusion via the peripheral veins may lead to local discomfort and inflammation. When repeated or continuous infusion is anticipated,
administration by a central venous catheter is recommended. Experience has shown that amiodarone can be absorbed into PVC infusion bags and administration sets possibly because of the presence of plasticisers in PVC plastic. It is important to prepare the infusion solution immediately prior to use in either glass or rigid PVC bottles containing no plasticisers. The use of medical equipment or devices containing plasticiser such as DEHP (di-2-ethylhexyl phthalate) in the presence of amiodarone injection may result in leaching out of DEHP. In order to minimise patient exposure to DEHB, the final amiodarone dilution for infusion may preferably be administered through non-DEHB containing sets.
Elderly Use :
As with all patients it is important the minimum effective dose is used. Whilst there is no evidence that dosage requirements are different for this group of patients they may be more susceptible to bradycardia and conduction defects if too high a dose is used. Particular
attention should be paid to monitoring of thyroid function.
CONTRAINDICATIONS :
Known hypersensitivity to Iodine or Amiodarone. Pregnancy and lactation. In patients in whom bradycardia or AV block is sufficient to cause syncope, patients with sick sinus syndrome (risk of sinus arrest) or with severe conduction disorders, CORDASOL should only be used in conjunction with a pacemaker. Sinus bradycardia and sino-atrial heart block. CORDASOL is contraindicated in patients with evidence, or a history of thyroid dysfunction. Combined therapy with drugs which may induce ‘Torsades de Pointes’.CORDASOL is contraindicated in the case of hypotension, severe respiratory failure, myocardiopathy, heart failure, circulatory collapse and severe arterial hypotension.
WARNINGS :
The ampoules of CORDASOL contain Benzyl Alcohol. There have been reports of fatal “gasping syndrome” in neonates (children less than one month of age) following the administration of intravenous solutions containing this preservative. Symptoms include a striking
onset of gasping syndrome, hypotension, bradycardia and cardiovascular collapse.
PRECAUTIONS :
It is recommended to perform an ECG and serum potassium measurement before treatment initiation. As Amiodarone may induce thyroid disorders, particularly in patients with personal or family history of thyroid disorders, clinical and biological monitoring is
recommended before starting treatment, ultrasensitive TSH (usTSH) assay, during treatment and for several months following treatment discontinuation. Serum usTSH levels should be measured when thyroid dysfunction is suspected. Regular monitoring of liver function tests (transaminases) is recommended during treatment.
Neurological toxicity : Peripheral neuropathy could occur in patients on long term high dosage (generally over 400 mg/day) regime. Intracellular inclusion bodies, similar to those seen in skin have been demonstrated in peripheral nerve fibres. Sensorimotor neuropathy, with a glove and stocking distribution have been reported in patients. Histologically, segmental demyelination of the nerve fibres has also been demonstrated. After discontinuation of the drug, the neurological complication is slowly and incompletely resolved.
Use in Renal Disease : Renal excretion of the drug is minimal. This suggests that modification of the dose of amiodarone in patients with renal failure is unnecessary.
Carcinogenic potential : In a carcinogenicity study in rats, amiodarone caused a dose related increase in thyroid follicular tumours (adenomas and/or carcinomas) in both sexes. Although mutagenicity findings were negative, an epigenic rather than genotoxic mechanism is proposed for this type of tumour induction. In the mouse, carcinomas were not observed but dose dependent thyroid follicular hyperplasia was seen. The relevance of these findings to man is unknown. Clinical experience has indicated that Amiodarone can effect thyroid function.
Pregnancy : Pregnancy Category C
Because of the long half-life of Amiodarone and its major metabolite and the potential to cause abnormal thyroid function and bradycardia in the foetus, its use is probably best avoided in the three months before and throughout the duration of pregnancy. Where exposure of the foetus is unavoidable, thyroid function (including TSH) should be assessed promptly in the newborn infant. No teratogenic effects have been observed in animals. The drug does cross the placenta. The long half-life of the drug requires that the drug be stopped several months before conception. The possible adverse effects of Amiodarone on the foetal thyroid are of concern since administration of iodine (of which there are 75 mg in a 200 mg dose of amiodarone) during pregnancy may cause foetal goitre, hypothyroidism and mental retardation. Amiodarone is contraindicated in pregnancy.

 Cardiovascular
Cardiovascular