1 mg/ml
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
PRANOSOL - P (Propranolol Hydrochloride) is a synthetic beta-adrenergic receptor blocking agent. Chemically, Propranolol Hydrochloride is (RS)-1-isopropylamino-3-(1-naphthyloxy)propan-2-ol hydrochloride. Its molecular formula is C16H21NO2,HCl and molecular weight is 295.81.
STRUCTURAL FORMULA :
Its structural formula is :
-Structure.jpg)
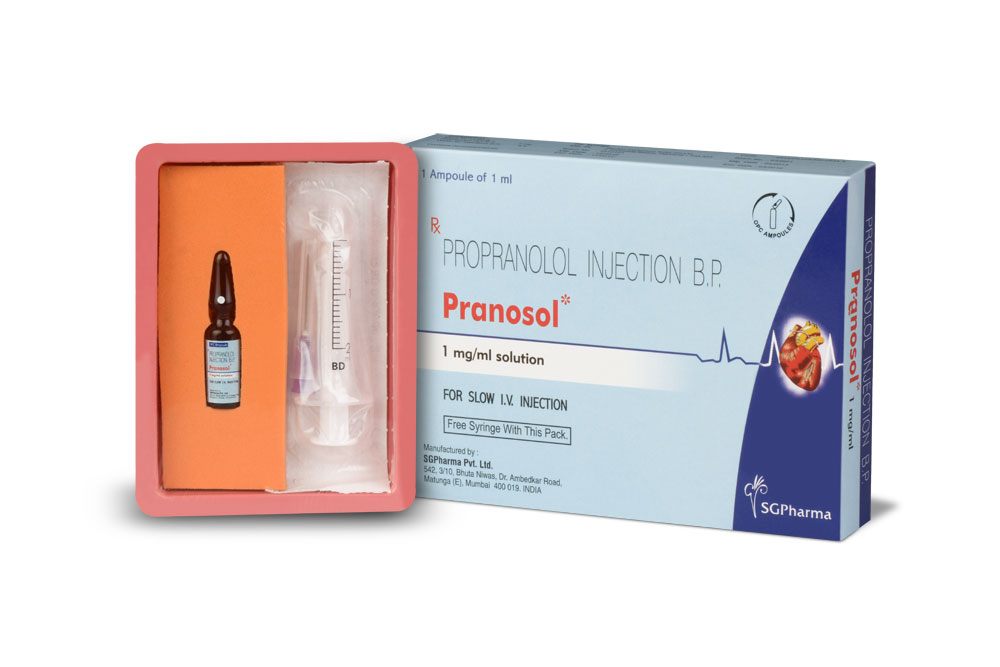
PRANOSOL - P is a sterile, clear, colourless solution filled in 1 ml amber glass ampoule.
COMPOSITION :
Each ml contains :
Propranolol Hydrochloride B.P. 1 mg
Water for Injections B.P. q.s.
Contains no preservatives.
ACTIONS :
Propranolol is a competitive antagonist at both the beta1 and beta2-adrenoreceptors. It has no agonist activity at the beta-adrenoreceptor but has membrane stabilising activity at concentrations exceeding 1 to 3 mg/l. Competitive beta-adrenoreceptor blockade has been demonstrated in man by a parallel shift to the right in the dose-heart rate response curve to beta-agonists such as isoprenaline. Propranolol, as with other beta-adrenoreceptor blocking medicines, has negative inotropic effects and is therefore contraindicated in uncontrolled heart failure. Propranolol is a racemic mixture and the active form is the S(-) isomer, of Propranolol. With the exception of inhibition of the conversion of thyroxine to triiodothyronine it is unlikely that any additional ancillary properties possessed by R(+) Propranolol, in comparison with the racemic mixture will give rise to different therapeutic effects. Propranolol is effective and well-tolerated in most ethnic populations, although the response may be less in Afro-Caribbean black patients.
PHARMACOKINETICS :
Following intravenous administration the plasma half-life of Propranolol is about 2 hours and the ratio of metabolites to parent medicine in the blood is lower than after oral administration. In particular 4-Hydroxy Propranolol is not present after intravenous administration.
Propranolol is widely and rapidly distributed throughout the body with highest levels occurring in the lungs, liver, kidney, brain and heart. Propranolol is highly protein bound (80 % to 95 %). The liver removes up to 90 % of an oral dose with an elimination half-life of 3-6 hours. Since the half-life may be increased in patients with significant hepatic or renal impairment, care should be taken when starting treatment and selecting the initial dose.
INDICATIONS :
Cardiac Arrhythmias :
1) Supraventricular arrhythmias
a) Paroxysmal atrial tachycardias, particularly those arrhythmias induced by catecholamines or digitalis or associated with the Wolff-Parkinson-White syndrome.
b) Persistent sinus tachycardia which is noncompensatory and impairs the wellbeing of the patient.
c) Tachycardias and arrhythmias due to thyrotoxicosis when causing distress or increased hazard and when immediate effect is necessary as adjunctive, short term (2 to 4 weeks) therapy. May be used with, but not in place of, specific therapy.
d) Persistent atrial extrasystoles which impair the well-being of the patient and do not respond to conventional measures.
e) Atrial flutter and fibrillation when ventricular rate cannot be controlled by digitalis alone, or when digitalis is contraindicated.
2) Ventricular tachycardias Ventricular arrhythmias do not respond to Propranolol as predictably as do the supraventricular arrhythmias.
a) Ventricular tachycardias. With the exception of those induced by catecholamines or digitalis, Propranolol is not the drug of first choice. In critical situations when cardioversion techniques or other drugs are not indicated or are not effective, Propranolol may be considered. If after consideration of the risks involved,Propranolol is used, it should be given intravenously in low dosage and very slowly. Care in the administration of Propranolol with constant electrocardiographic monitoring is essential as the failing heart requires some sympathetic drive for maintenance of myocardial tone.
b) Persistent premature ventricular extrasystoles which do not respond to conventional measures and impair the well-being of the patient.
3) Tachyarrhythmias of digitalis intoxication If digitalis-induced tachyarrhythmias persist following discontinuance of digitalis and correction of lectrolyte abnormalities, they are usually reversible with oral Propranolol. Severe bradycardia may occur. Intravenous Propranolol hydrochloride is reserved for life-threatening arrhythmias. Temporary maintenance with oral therapy may be indicated.
4) Resistant tachyarrhythmias due to excessive catecholamine action during anaesthesia. Tachyarrhythmias due to excessive catecholamine action during anaesthesia may sometimes arise because of release of endogenous catecholamines or administration of catecholamines. When usual measures fail in such arrhythmias, Propranolol may be given intravenously to abolish them. All general inhalation anaesthetics produce some degree of myocardial depression. Therefore, when Propranolol is used to treat arrhythmias during anaesthesia, it should be used with extreme caution and constant ECG and central venous pressure monitoring.
Administration :
By Intravenous Injection.
INSTRUCTION FOR USE OF AMPOULE :
The ampoule used in this product is equipped with O.P.C (One Point Cut) opening system. No ampoule file is needed to open the ampoule. The neck of the ampoule is prescored at the point of constriction. A coloured dot on the ampoule head helps to orientate the ampoule. Take the ampoule and face the coloured dot. Let the solution at the head of the ampoule to flow down by shaking or a gentle stroke. The ampoule opens easily by placing the thumb on the coloured dot and gently pressing downwards as shown.

Dosage :
Adults :
The initial dose of PRANOSOL - P is 1 mg (1 ml) injected over 1 minute. This may be repeated at 2-minute intervals until a response is observed or to a maximum dose of 10 mg in conscious patients or 5 mg in patients under anaesthesia.
Elderly :
Evidence concerning the relation between blood level and age is conflicting. PRANOSOL - P should be used to treat the elderly with caution. It is suggested that treatment should start with the lowest dose. The optimum dose should be individually determined according to clinical response.
Children :
Dysrhythmias, thyrotoxicosis :
Dosage should be individually determined and the following is only a guide :
Intravenous : 0.025 to 0.05 mg/kg injected slowly under ECG control and repeated 3 or 4 times daily as required.
Fallot’s tetralogy :
The value of PRANOSOL - P in this condition is confined mainly to the relief of right-ventricular outflow tract shut-down. It is also useful for treatment of associated dysrhythmias and angina. Dosage should be individually determined and the following is only a guide :
Intravenous : Up to 0.1 mg/kg injected slowly under ECG control, repeated 3 or 4 times daily as required.
CONTRAINDICATIONS :
PRANOSOL - P must not be used if there is a history of bronchial asthma or bronchospasm.
PRANOSOL - P as with other beta-adrenoreceptor blocking medicines must not be used in patients with any of the following :
- Known hypersensitivity to the substance
- Bradycardia
- Cardiogenic shock
- Hypotension
- Metabolic acidosis
- After prolonged fasting
- Severe peripheral arterial circulatory disturbances
- Second or third degree heart block
- Sick sinus syndrome
- Untreated phaeochromocytom
- Uncontrolled heart failure
- Prinzmetal’s angina.
PRANOSOL - P must not be used in patients prone to hypoglycaemia i.e. Patients after prolonged fasting or patients with restricted counter-regulatory reserves.
WARNINGS :
Cardiac Failure :
Sympathetic stimulation may be vital component supporting circulatory function in patients with congestive heart failure and its inhibition by beta blockade may precipitate more severe failure. Although beta blockers should be avoided in overt congestive heart failure, if necessary, they can be used with close follow-up in patients with a history of failure who are well compensated and are receiving digitalis and diuretics. Beta-adrenergic blocking agents do not abolish the inotropic action of digitalis on heart muscle. In Patients Without a History of Heart Failure, continued use of beta blockers can, in some cases, lead to cardiac failure. Therefore, at the first sign or symptom of heart failure, the patient should be digitalized and/or treated with diuretics the response observed closely or Propranolol should be discontinued (gradually, if possible).
Pregnancy :
As with all other medicines, PRANOSOL - P should not be given in pregnancy unless its use is essential. There is no evidence of teratogenicity with Propranolol HCl Injection. However, beta-adrenoreceptor blocking medicines reduce placental perfusion, which may result in intra-uterine foetal death, immature and premature deliveries. In addition, adverse effects (especially hypoglycaemia and bradycardia in the neonate and bradycardia in the foetus) may occur. There is an increased risk of cardiac and pulmonary complications in the neonate in the post-natal period.
Lactation :
Most beta-adrenoreceptor blocking medicines, particularly lipophilic compounds, will pass into breast milk although to a variable extent. Breast feeding is therefore not recommended following administration of these compounds.
INTERACTIONS :
PRANOSOL - P modifies the tachycardia of hypoglycaemia. Caution must be exercised in the concurrent use of PRANOSOL - P and hypoglycaemic therapy in diabetic patients. Propranolol HCl Injection may prolong the hypoglycaemic response to insulin. Caution must be exercised in prescribing a beta-adrenoceptor blocking drug with Class I antiarrhythmic agents such as disopyramide. Digitalis glycosides in association with beta-adrenoceptor blocking drugs may increase atrioventricular conduction time. Combined use of beta-adrenoceptor blocking drugs and calcium channel blockers with negative inotropic effects (e.g. verapamil, diltiazem) can lead to an exaggeration of these effects particularly in patients with impaired ventricular function and/or SA or AV conduction abnormalities. This may result in severe hypotension, bradycardia and cardiac failure. Neither the beta-adrenoceptor blocking drug nor the calcium channel blocker should be administered intravenously within 48 hours of discontinuing the other. Concomitant therapy with dihydropyridines calcium channel blockers e.g. nifedipine, may increase the risk of hypotension and cardiac failure may occur in patients with latent cardiac insufficiency.
Concomitant use of sympathomimetic agents e.g. adrenaline, may counteract the effect of beta-adrenoceptor blocking drugs. Caution must be exercised in the parenteral administration of preparations containing adrenaline to patients taking beta-adrenoceptor blocking drugs as, in rare cases, vasoconstriction, hypertension and bradycardia may result. Administration of Propranolol HCl Injection during infusion of lignocaine may increase the plasma concentration of lignocaine by approximately 30 %. Patients already receiving Propranolol tend to have higher lignocaine levels than controls. The combination should be avoided. Concomitant use of cimetidine or hydralazine will increase, whereas concomitant use of alcohol will decrease, the plasma levels of Propranolol. Beta-adrenoceptor blocking drugs may exacerbate the rebound hypertension which can follow the withdrawal of clonidine. If the two drugs are co-administered, the beta-adrenoceptor blocking drug should be withdrawn several days before discontinuing clonidine. If replacing clonidine by beta-adrenoceptor blocking drug therapy, the introduction of beta-adrenoceptor blocking drugs should be delayed for several days after clonidine administration has stopped.
Caution must be exercised if ergotamine, dihydroergotamine or related compounds are given in combination with Propranolol since vasospastic reactions have been reported in a few patients. Concomitant use of prostaglandin synthetase inhibiting drugs e.g. ibuprofen and indomethacin, may decrease the hypotensive effects of Propranolol HCl Injection.Concomitant administration of Propranolol HCl Injection and chlorpromazine may result in an increase in plasma levels of both drugs. This may lead to an enhanced antipsychotic effect for chlorpromazine and an increased antihypertensive effect for Propranolol HCl Injection.Caution must be exercised when using anaesthetic agents with Propranolol HCl Injection. The anaesthetist should be informed and the choice of anaesthetic should be an agent with as little negative inotropic activity as possible. Use of beta-adrenoceptor blocking drugs with anaesthetic drugs may result in attenuation of the reflex tachycardia and increase the risk of hypotension. Anaesthetic agents causing myocardial depression are best avoided. Pharmacokinetic studies have shown that the following agents may interact with propranolol due to effects on enzyme systems in the liver which metabolise propranolol and these agents : quinidine, propafenone, rifampicin, theophylline, warfarin, thioridazine and dihydropyridine calcium channel blockers such as nifedipine, nisoldipine, nicardipine, isradipine and lacidipine. Owing to the fact that blood concentrations of either agent may be affected dosage adjustments may be needed according to clinical judgement.
SIDE EFFECTS :
Most adverse effects have been mild and transient and have rarely required the withdrawal of therapy.
Cardiovascular : Bradycardia; congestive heart failure; intensification of AV block; hypotension; paresthesia of hands; thrombocytopenic purpura; arterial insufficiency, usually of the Raynaud type.
Central Nervous System : Lightheadedness; mental depression manifested by insomnia, lassitude, weakness, fatigue; reversible mental depression progressing to catatonia; visual disturbances; hallucinations, vivid dreams, an acute reversible syndrome characterized by disorientation for time and place, short-term memory loss, emotional liability, slightly clouded sensorium and decreased performance on neuropsychometrics. Total oral daily doses above 160 mg (when administered as divided doses of greater than 80 mg each) may be associated with an increased incidence of fatigue, lethargy and vivid dreams.
Gastrointestinal : Nausea, vomiting, epigastric distress, abdominal cramping, diarrhoea, constipation, mesenteric arterial thrombosis, ischemic colitis.
Allergic : Pharyngitis and agranulocytosis, erythematous rash, fever combined with aching and sore throat, laryngospasm and respiratory distress.
Respiratory : Bronchospasm.
Haematologic : Agranulocytosis, nonthrombocytopenic purpura, thrombocytopenic purpura.
Autoimmune : In extremely rare instances, systemic lupus erythematosus has been reported.
Miscellaneous : Alopecia, LE-like reactions, psoriasiform rashes, dry eyes, male impotence and Peyronie’s disease have been reported rarely. Oculomucocutaneous reactions involving the skin, serous membranes and conjunctivae reported for a beta blocker (practolol) have not been associated with propranolol.
TREATMENT OF OVERDOSAGES :
Bradycardia : Administer atropine (0.25 mg to 1.0 mg); if there is no response to vagal blockade, administer Isoproterenol cautiously.

 Cardiovascular
Cardiovascular