10 mg
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
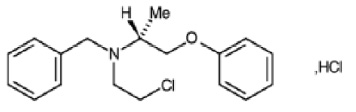
PHENOXYBENZAMINE CAPSULES B.P. (Phenoxybenzamine Hydrochloride) is a non-selective alpha-adrenoceptor antagonist. Chemically, Phenoxybenzamine Hydrochloride is (RS)-benzyl (2-chloroethyl) 1-methyl-2-phenoxyethylamine hydrochloride.The molecular formula is C18H22ClNO.HCl and molecular weight is 340.3.
STRUCTURAL FORMULA :
Its structural formula is :
PHENOXYBENZAMINE CAPSULES B.P. contains white or nearly white powder filled in hard gelatin capsule of suitable size.
COMPOSITION :
Each hard gelatin capsule contains :
Phenoxybenzamine Hydrochloride B.P. 10 mg
Excipients q.s.
Approved colours used in empty capsule shell.
ACTIONS :
Phenoxybenzamine Hydrochloride is a long-acting, adrenergic, alpha -receptor blocking agent which can produce and maintain “chemical sympathectomy” by oral administration. It increases blood flow to the skin, mucosa and abdominal viscera, and lowers both supine and erect blood pressures. It has no effect on the parasympathetic system.
PHARMACOKINETICS :
Phenoxybenzamine is incompletely and variably absorbed from the gastrointestinal tract. After oral dosage the onset of action is gradual over several hours and persists for 3 or 4 days following a single dose. The maximum effect is attained in about 1 hour after an intravenous dose. The plasma half-life after intravenous dosage is about 24 hours. Phenoxybenzamine is metabolised in the liver and excreted in the urine and bile, but small amounts remain in the body for several days. The duration of action is thought to depend on the rate of synthesis of new alpha receptors following irreversible covalent bonding to existing alpha receptors by a reactive intermediate of phenoxybenzamine.
INDICATIONS :
Pheochromocytoma : PHENOXYBENZAMINE CAPSULES B.P. is indicated to control episodes of hypertension and sweating in the treatment of pheochromocytoma as preoperative preparation for surgery, in management of patients when surgery is contraindicated, and in chronic management of patients with malignant pheochromocytoma.
Benign prostatic hypertrophy : PHENOXYBENZAMINE CAPSULES B.P. is used for the treatment of urinary symptoms associated with benign prostatic hypertrophy (BPH)
Administration :
FOR ORAL USE
Dosage :
Usual adult dose Antihypertensive (pheochromocytoma) - Initial : Oral, 10 mg twice a day, increased by increments of 10 mg every other day until an adequate response is achieved.
Maintenance : Oral, 20 to 40 mg two or three times a day. [Benign prostatic hypertrophy therapy] - 10 to 20 mg per day.
Note : Geriatric patients may be more sensitive to the effects of the usual adult dose.
Usual paediatric dose
Antihypertensive (pheochromocytoma) -
Initial : Oral, 200 mcg (0.2 mg) per kg of body weight or 6 mg per square meter of body surface, up to a maximum dose of 10 mg, once a day. Dosage is increased gradually at four-day intervals, until an adequate response is achieved.
Maintenance : Oral, 400 mcg (0.4 mg) to 1.2 mg per kg of body weight or 12 to 36 mg per square meter of body surface a day in three or four divided doses.
CONTRAINDICATIONS :
Conditions where a fall in blood pressure may be undesirable; hypersensitivity to the drug or any of its components. Do not use in patients who have had a cerebrovascular accident; or in the recovery period (usually 3-4 weeks) after acute myocardial infarction.
PHENOXYBENZAMINE CAPSULES B.P. contains lactose which is contra-indicated in patients with galactosaemia, the glucose-galactose malabsorption syndrome, or lactase deficiency.
WARNINGS :
PHENOXYBENZAMINE CAPSULES B.P. induced alpha-adrenergic blockade leaves beta-adrenergic receptors unopposed. Compounds that stimulate both types of receptors may, therefore, produce an exaggerated hypotensive response and tachycardia.
PRECAUTIONS :
General :
Administer with caution in patients with marked cerebral or coronary arteriosclerosis or renal damage. Adrenergic blocking effect may aggravate symptoms of respiratory infections. PHENOXYBENZAMINE CAPSULES B.P. should be used cautiously in diabetic patients.
Pregnancy : Category C
Adequate reproductive studies in animals have not been performed with phenoxybenzamine hydrochloride. It is also not known whether PHENOXYBENZAMINE CAPSULES B.P. can cause foetal harm when administered to a pregnant woman. PHENOXYBENZAMINE CAPSULES B.P. should be given to a pregnant woman only if clearly needed.
Nursing mothers :
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, and because of the potential for serious adverse reactions from phenoxybenzamine hydrochloride, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Paediatric Use :
Safety and effectiveness in paediatric patients have not been established.
INTERACTIONS AND INCOMPATIBILITIES :
Phenoxybenzamine hydrochloride may interact with compounds that stimulate both alpha- and beta-adrenergic receptors (i.e., epinephrine) to produce an exaggerated hypotensive response and tachycardia. PHENOXYBENZAMINE CAPSULES B.P. blocks hyperthermia production by levarterenol, and blocks hypothermia production by reserpine.
SIDE EFFECTS :
The following side effects have been observed, but there are insufficient data to support an estimate of their frequency. Autonomic Nervous System : Postural hypotension, tachycardia, inhibition of ejaculation, nasal congestion, miosis. These so-called “side effects” are actually evidence of adrenergic blockade and vary according to the degree of blockade. Miscellaneous : Gastrointestinal irritation, drowsiness, fatigue.
OVERDOSAGE :
Symptoms :
These are largely the result of blocking of the sympathetic nervous system and of the circulating epinephrine. They may include postural hypotension, resulting in dizziness or fainting; tachycardia, particularly postural; vomiting; lethargy; shock.
TREATMENT OF OVERDOSAGE :
When symptoms and signs of overdosage exist, discontinue the drug. Treatment of circulatory failure, if present, is a prime consideration. In cases of mild overdosage, recumbent position with legs elevated usually restores cerebral circulation. In the more severe cases, the usual measures to combat shock should be instituted. Usual pressor agents are not effective. Epinephrine is contraindicated because it stimulates both alpha- and beta- receptors; since alpha- receptors are blocked, the net effect of epinephrine administration is vasodilation and a further drop in blood pressure (epinephrine reversal). The patient may have to be kept flat for 24 hours or more in the case of overdose, as the effect of the drug is prolonged. Leg bandages and an abdominal binder may shorten the period of disability.
I.V. Infusion of levarterenol bitartrate may be used to combat severe hypotensive reactions, because it stimulates alpha-receptors primarily. Although phenoxybenzamine hydrochloride is an alpha -adrenergic blocking agent, a sufficient dose of levarterenol bitartrate will overcome this effect. The oral LD50 for phenoxybenzamine hydrochloride is approximately 2000 mg/kg in rats and approximately 500 mg/kg in guinea pigs.
STORAGE :
Store below 30°C (86°F), protected from moisture and light.
Do not refrigerate.
SHELF LIFE :
24 months from the date of manufacture.
PRESENTATION :
PHENOXYBENZAMINE CAPSULES B.P. contains Phenoxybenzamine Hydrochloride B.P. 10 mg.
3 Blisters of 10 Capsules Per Box.
Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.

 Cardiovascular
Cardiovascular