
25 mg/5 ml
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
DILTIAZEM INJECTION I.P. (Diltiazem Hydrochloride) is antianginal (calcium-channel blocker), antihypertensive, antiarrythmic (class IV). Chemically, Diltiazem Hydrochloride is 1,5-Benzothiazepin-4(5H)-one, 3-(acetyloxy)-5-[2-(dimethylamino)ethyl]-2,3-dihydro-2-(4-methoxyphenyl)-, monohydrochloride, (+)-cis-. The molecular formula is C22H26N2O4S ·HCl and molecular weight is 450.98.
STRUCTURAL FORMULA :
Its structural formula is :
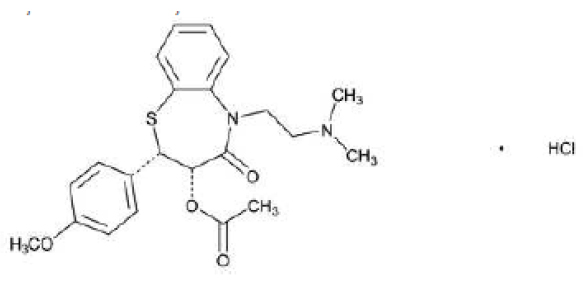
DILTIAZEM INJECTION I.P. is a clear, colourless solution filled in amber ampoule of suitable size.
COMPOSITION :
Each ml contains :
Diltiazem Hydrochloride USP 5 mg
Sorbitol Solution USP 71.4 mg
Water for Injection USP q.s.
Contains no preservatives.
ACTIONS :
Diltiazem inhibits the influx of calcium (Ca2+) ions during membrane depolarization of cardiac and vascular smooth muscle. The therapeutic benefits of diltiazem in supraventricular tachycardias are related to its ability to slow AV nodal conduction time and prolong AV nodal refractoriness. Diltiazem exhibits frequency (use) dependent effects on AV nodal conduction such that it may selectively reduce the heart rate during tachycardias involving the AV node with little or no effect on normal AV nodal conduction at normal heart rates.
Diltiazem slows the ventricular rate in patients with a rapid ventricular response during atrial fibrillation or atrial flutter. Diltiazem converts paroxysmal supraventricular tachycardia (PSVT) to normal sinus rhythm by interrupting the reentry circuit in AV nodal reentrant tachycardias and reciprocating tachycardias, eg. Wolff-Parkinson-White syndrome (WPW).
Diltiazem prolongs the sinus cycle length. It has no effect on the sinus node recovery time or on the sinoatrial conduction time in patients without SA nodal dysfunction. Diltiazem has no significant electrophysiologic effects on tissues in the heart that are fast sodium
channel dependent, eg, His-Purkinje tissue, atrial and ventricular muscle, and extranodal accessory pathways. Like other calcium antagonists, because of its effect on vascular smooth muscle, diltiazem decreases total peripheral resistance resulting in a decrease in both systolic and diastolic blood pressure.
Haemodynamics :
In patients with cardiovascular disease, diltiazem hydrochloride administered intravenously in single bolus doses, followed in some cases by a continuous infusion, reduced blood pressure, systemic vascular resistance, the rate-pressure product, and coronary vascular resistance and increased coronary blood flow. In a limited number of studies of patients with compromised myocardium (severe congestive heart failure, acute myocardial infarction, hypertrophic cardiomyopathy), administration of intravenous diltiazem produced no significant effect on contractility, left ventricular end diastolic pressure, or pulmonary capillary wedge pressure. The mean ejection fraction and cardiac output/index remained unchanged or increased. Maximal haemodynamic effects usually occurred within 2 to 5 minutes of an injection. However, in rare instances, worsening of congestive heart failure has been reported in patients with pre-existing impaired ventricular function.
Pharmacodynamics :
The prolongation of PR interval correlated significantly with plasma diltiazem concentration in normal volunteers using the Sigmoidal Emax model. Changes in heart rate, systolic blood pressure, and diastolic blood pressure did not correlate with diltiazem plasma concentration in normal volunteers. Reduction in mean arterial pressure correlated linearly with diltiazem plasma concentration in a group of hypertensive patients. In patients with atrial fibrillation and atrial flutter, a significant correlation was observed between the percent reduction in HR and plasma diltiazem concentration using the Signmoidal Emax model. Based on this relationship, the mean plasma diltiazem concentration required to produce a 20 % decrease in heart rate was determined to be 80 ng/ml. Mean plasma diltiazem concentrations of 130 ng/ml and 300 ng/ml were determined to produce reductions in heart rate of 30 % to 40 %.
PHARMACOKINETICS :
Following a single intravenous injection in healthy male volunteers, diltiazem hydrochloride appears to obey linear pharmacokinetics over a dose range of 10.5 to 21 mg. The plasma elimination half-life is approximately 3.4 hours. The apparent volume of distribution of diltiazem hydrochloride is approximately 305 L. Diltiazem is extensively metabolized in the liver with a systemic clearance of approximately 65 L/h. After constant rate intravenous infusion to healthy male volunteers, diltiazem exhibits nonlinear pharmacokinetics over an infusion range of 4.8 to 13.2 mg/h for 24 hours. Over this infusion range, as the dose is increased, systemic clearance decreases from 64 to 48 L/h while the plasma elimination half-life increases from 4.1 to 4.9 hours. The apparent volume of distribution remains unchanged (360 to 391 L). In patients with atrial fibrillation or atrial flutter, diltiazem systemic clearance has been found to be decreased compared to healthy volunteers. In patients administered bolus doses ranging from 2.5 mg to 38.5 mg, systemic clearance averaged 36 L/h. In patients administered continuous infusions at 10 mg/h or 15 mg/h for 24 hours, diltiazem systemic clearance averaged 42 L/h and 31 L/h, respectively.
Based on the results of pharmacokinetic studies in healthy volunteers administered different oral diltiazem hydrochloride formulations, constant rate intravenous infusions of diltiazem hydrochloride at 3, 5, 7, and 11 mg/h are predicted to produce steady-state plasma diltiazem concentrations equivalent to 120-, 180-, 240-, and 360-mg total daily oral doses of diltiazem hydrochloride tablets or capsules. After oral administration, diltiazem hydrochloride undergoes extensive metabolism in man by deacetylation, N-demethylation, and O-demethylation via cytochrome P-450 (oxidative metabolism) in addition to conjugation. Metabolites N-monodesmethyldiltiazem, desacetyldiltiazem, desacetyl-N-monodesmethyldiltiazem, desacetyl-O-desmethyldiltiazem, and desacetyl-N, O-desmethyldiltiazem have been identified in human urine. Following oral administration, 2 % to 4 % of the unchanged diltiazem appears in the urine. Drugs which induce or inhibit hepatic microsomal enzymes may alter diltiazem disposition.
Following single intravenous injection of diltiazem hydrochloride, however, plasma concentrations of N-monodesmethyldiltiazem and desacetyldiltiazem, two principal metabolites found in plasma after oral administration, are typically not detected. These metabolites are observed, however, following 24 hour constant rate intravenous infusion. Total radioactivity measurement following short IV administration in healthy volunteers suggests the presence of other unidentified metabolites which attain higher concentrations than those of diltiazem and are more slowly eliminated; half-life of total radioactivity is about 20 hours compared to 2 to 5 hours for diltiazem. Diltiazem hydrochloride is 70 % to 80 % bound to plasma proteins. In vitro studies suggest alpha1-acid glycoprotein binds approximately 40 % of the drug at clinically significant concentrations. Albumin appears to bind approximately 30 % of the drug, while other constituents bind the remaining bound fraction. Competitive in vitro ligand binding studies have shown that diltiazem binding is not altered by therapeutic concentrations of digoxin, phenytoin, hydrochlorothiazide, indomethacin, phenylbutazone, propranolol, salicylic acid, tolbutamide, or warfarin. Renal insufficiency, or even end-stage renal disease, does not appear to influence diltiazem disposition following oral administration. Liver cirrhosis was shown to reduce diltiazem’s apparent oral clearance and prolong its half-life.
INDICATIONS :
DILTIAZEM INJECTION I.P. is indicated for the following :
Atrial Fibrillation or Atrial Flutter :
Temporary control of rapid ventricular rate in atrial fibrillation or atrial flutter. It should not be used in patients with AF/FL associated with an accessory bypass tract such as in Wolff-Parkinson-White (WPW) syndrome, or short PR syndrome, e.g. Lown-Ganong-Levine syndrome. DILTIAZEM INJECTION I.P. rarely converts atrial fibrillation or atrial flutter to normal sinus rhythm.
Paroxysmal Supraventricular Tachycardia :
Rapid conversion of paroxysmal supraventricular tachycardias to sinus rhythm. This includes AV nodal re-entrant tachycardias and reciprocating tachycardias associated with an extra-nodal accessory pathway, such as the WPW syndrome, or short PR syndrome, e.g. Lown-Ganong-Levine syndrome. Unless otherwise contraindicated, appropriate vagal manoeuvres should be attempted prior to administration of DILTIAZEM INJECTION I.P. The use of DILTIAZEM INJECTION I.P. for control of ventricular response in patients with atrial fibrillation or atrial flutter or conversion to sinus rhythm in patients with paroxysmal supraventricular tachycardia should be undertaken with caution when the patient is compromised haemodynamically or is taking other drugs that decrease any or all of the following : peripheral resistance, myocardial filling, myocardial contractility, or electrical impulse propagation in the myocardium. For either indication the setting should include continuous monitoring of the ECG and frequent measurement of blood pressure. A defibrillator and emergency equipment should be readily available.
Administration :
DILTIAZEM INJECTION I.P. is given by direct intravenous bolous injection and continuous intravenous infusion.
INSTRUCTIONS FOR USE OF AMPOULE :
The ampoule used in this product is equipped with O.P.C. (One Point Cut) opening system. No ampoule file is needed to open the ampoule. The neck of the ampoule is prescored at the point of constriction. A coloured dot on the ampoule head helps to orientate the ampoule. Take the ampoule and face the coloured dot. Let the solution at the head of the ampoule to flow down by shaking or a gentle stroke. The ampoule opens easily by placing the thumb on the coloured dot and gently pressing downwards as shown.

Dosage :
The initial dose of DILTIAZEM INJECTION I.P. should be 0.25 mg/kg body weight as a bolus administered over 2 minutes. If response is inadequate, a second dose may be administered after 15 minutes. The second bolus dose of DILTIAZEM INJECTION I.P. should be 0.35 mg/kg body weight administered over two minutes. Subsequent intravenous bolus doses should be individualized for each patient. Some patients may respond to an initial dose of 0.15 mg/kg, although duration of action may be shorter.
Continuous Intravenous Infusion :
For continued reduction of the heart rate (up to 24 hours) in patients with atrial fibrillation or atrial flutter, an intravenous infusion of DILTIAZEM INJECTION I.P. may be administered. Immediately following bolus administration of 20 mg (0.25 mg/kg) or 25 mg (0.35 mg/kg) DILTIAZEM INJECTION I.P. and reduction of heart rate, begin an intravenous infusion of DILTIAZEM INJECTION I.P. The recommended initial infusion rate of DILTIAZEM INJECTION I.P. is 10 mg/h. Some patients may maintain response to an initial rate of 5 mg/h. The infusion rate may be increased in 5 mg/h increments up to 15 mg/h as needed, if further reduction in heart rate is required, the infusion may be maintained for up to 24 hours. Diltiazem shows dose-dependent, non-linear pharmacokinetics. Duration of infusion longer than 24 hours and infusion rates greater than 15 mg/h have not been studied. Therefore, infusion duration exceeding 24 hours and infusion rates exceeding 15 mg/h are not recommended.
CONTRAINDICATIONS :
DILTIAZEM INJECTION I.P. is contraindicated in :
- Patients with sick sinus syndrome except in the presence of a functioning ventricular pacemaker.
- Patients with second- or third-degree AV block except in the presence of functioning ventricular pacemaker.
- Patients with severe hypotension or cardiogenic shock.
- Patients who have demonstrated hypersensitivity to the drug.
- Intravenous diltiazem and intravenous beta-blockers should not be administered together or in close proximity (within a few hours).
- Patients with atrial fibrillation or atrial flutter associated with an accessory bypass tract such as in WPW syndrome or short PR syndrome.
As with other agents which slow AV nodal conduction and do not prolong the refractoriness of the accessory pathway (eg, verapamil, digoxin), in rare instances patients in atrial fibrillation or atrial flutter associated with an accessory bypass tract may experience a potentially life-threatening increase in heart rate accompanied by hypotension when treated DILTIAZEM INJECTION I.P. As such, the initial use of DILTIAZEM INJECTION I.P. should be, if possible, in a setting where monitoring and resuscitation capabilities, including DC cardioversion/defibrillation, are present. Once familiarity of the patient’s response is established, use in an office setting may be acceptable.
- Patients with ventricular tachycardia. Administration of other calcium channel blockers to patients with wide complex tachycardia
- In pregnancy and in women of childbearing potential.
WARNINGS :
Cardiac Conduction :
Diltiazem prolongs AV nodal conduction and refractoriness that may rarely result in second- or third-degree AV block or sinus rhythm. Concomitant use of diltiazem with agents known to affect cardiac conduction may result in additive effects. If high-degree AV block occurs in sinus rhythm, intravenous diltiazem should be discontinued and appropriate supportive measures instituted.
Congestive Heart Failure :
Although diltiazem has a negative inotropic effect in isolated animal tissue preparations, haemodynamic studies in humans with normal ventricular function and in patients with a compromised myocardium, such as severe CHF, acute MI, and hypertrophic cardiomyopathy, have not shown a reduction in cardiac index nor consistent negative effects on contractility (dp/dt). Administration of oral diltiazem in patients with acute myocardial infarction and pulmonary congestion documented by x-ray on admission is contraindicated. Experience with the use of DILTIAZEM INJECTION I.P. in patients with impaired ventricular function is limited. Caution should be exercised when using the drug in such patients.
Hypotension :
Decreases in blood pressure associated with DILTIAZEM INJECTION I.P. therapy may occasionally result in symptomatic hypotension (3.2 %). The use of intravenous diltiazem for control of ventricular response in patients with supraventricular arrhythmias should be undertaken with caution when the patient is compromised haemodynamically. In addition, caution should be used in patients taking other drugs that decrease peripheral resistance, intravascular volume, myocardial contractility or conduction.
Acute Hepatic Injury :
In rare instances, significant elevations in enzymes such as alkaline phosphatase, LDH, SGOT, SGPT, and other phenomena consistent with acute hepatic injury have been noted following oral diltiazem. Therefore, the potential for acute hepatic injury exists following administration of intravenous diltiazem. Ventricular Premature Beats (VPBs) : VPBs may be present on conversion of PSVT to sinus rhythm with DILTIAZEM INJECTION I.P. These VPBs are transient, are typically considered to be benign, and appear to have no clinical significance. Similar ventricular complexes have been noted during cardioversion, other pharmacologic therapy, and during spontaneous conversion of PSVT to sinus rhythm.
PRECAUTIONS :
Dermatologic Events :
Dermatologic events may be transient and may disappear despite continued use of diltiazem. Dermatologic events progressing to erythema multiform and/or exfoliative dermatitis have been infrequently reported following oral diltiazem. Therefore the potential for these dermatologic reactions exists following exposure to intravenous diltiazem. Should a dermatologic reaction persist, the drug should be discontinued.
Impaired Hepatic or Renal Function :
Diltiazem is extensively metabolized by the liver and excreted by the kidneys and in bile. The drug should be used with caution in patients with impaired renal or hepatic function. The monitoring of laboratory parameters of renal or hepatic function is recommended. Liver cirrhosis was shown to reduce apparent oral diltiazem clearance, prolong the half-life of orally administered diltiazem and increase its bioavailability by 69 %.
Pregnancy : Category C
Well-controlled studies in humans have not been done. Studies in mice, rats, and rabbits, using doses of diltiazem 5 to 10 times greater than the recommended daily dose on a mg/kg basis, resulted in embryo and foetal deaths, reduced neonatal survival rates, and skeletal abnormalities. In addition, there was an increased incidence of stillbirths at doses of 20 or more times the recommended human dose.
Nursing mothers :
Diltiazem is distributed into breast milk, reaching concentrations approximate to maternal serum concentrations. If use of diltiazem is deemed essential, an alternative method of infant feeding should be instituted.
Paediatric Use :
Safety and effectiveness in paediatric patients have not been established.
INTERACTIONS :
Due to the potential for additive effects, caution is warranted in patients receiving diltiazem concomitantly with any agent(s) known to affect cardiac contractility and/or SA or AV node conduction.
Cytochrome P450 System :
As with all drugs, care should be exercised when treating patients with multiple medications. Calcium channel blockers undergo biotransformation by the cytochrome P450 system. Coadministration of diltiazem with other drugs which follow the same route of biotransformation may result in altered bioavailability. Dosages of similarly metabolized drugs, particularly those of low therapeutic ratio, and especially in patients with renal and/or hepatic impairment, may require adjustment when starting or stopping concomitantly administered diltiazem to maintain optimum therapeutic blood levels. Drugs known to be inhibitors of the cytochrome P450 system include : azole antifungals, cimetidine, cyclosporine, erythromycin, quinidine, and warfarin. Drugs known to be inducers of the cytochrome P450 system include : phenobarbital, phenytoin, and rifampin. Drugs known to be biotransformed via P450 include : benzodiazepines, flecainide, imipramine, propafenone, terfenadine, and theophylline.
Amiodarone :
Severe conduction system abnormalities including heart block of varying degree, sinus arrest and low cardiac output state of life-threatening severity have been reported following concomitant use of diltiazem and amiodarone. These drugs may also have additive effects on cardiac conduction and contractility.
Anaesthetics :
The depression of cardiac contractility, conductivity, and automaticity as well as the vascular dilation associated with anaesthetics may be potentiated by calcium channel blockers. When used concomitantly, anaesthetics and calcium blockers should be titrated carefully.
Benzodiazepines :
Diltiazem significantly increases peak plasma levels and the elimination half-life of triazolam and midazolam.
Beta-Blockers :
Intravenous diltiazem has been administered to patients on chronic oral beta-blocker therapy. The combination of the two drugs was generally well tolerated without serious adverse effects. If intravenous diltiazem is administered to patients receiving chronic oral beta-blocker therapy, the possibility for bradycardia, AV block and/or depression of contractility should be considered.
Calcium Antagonists :
Limited clinical experience suggests that in certain severe conditions not responding adequately to verapamil or to nifedipine, using diltiazem in conjunction with either of these drugs may be beneficial.
Carbamazepine :
Concomitant administration of oral diltiazem with carbamazepine has been reported to result in elevated serum levels of carbamazepine (40 % to 72 % increase) resulting in toxicity in some cases. Patients receiving these drugs concurrently should be monitored for a potential drug interaction.
Cimetidine :
A study in six healthy volunteers has shown a significant increase in peak diltiazem plasma levels (58 %) and area-under-the-curve (53 %) after a 1-week course of cimetidine at 1200 mg per day and a single dose of oral diltiazem 60 mg. Ranitidine produced smaller, nonsignificant increases. Patients currently receiving diltiazem therapy should be carefully monitored for a change in pharmacological effect when initiating and discontinuing therapy with cimetidine. An adjustment in the diltiazem dose may be warranted.
Cyclosporine :
A pharmacokinetic interaction between diltiazem and cyclosporine has been observed during studies involving renal and cardiac transplant patients. In renal and cardiac transplant recipients, a reduction of cyclosporine dose ranging from 15 % to 48 % was necessary to maintain cyclosporine through concentrations similar to those seen prior to the addition of diltiazem. If these agents are to be administered concurrently, cyclosporine concentrations should be monitored, especially when diltiazem therapy is initiated, adjusted or discontinued. The effect of cyclosporine on diltiazem plasma concentrations has not been evaluated.
Digitalis :
Intravenous diltiazem has been administered to patients receiving either intravenous or oral digitalis therapy. The combination of the two drugs was well tolerated without serious adverse effects. However, since both drugs affect AV nodal conduction, patients should be monitored for excessive slowing of the heart rate and/or AV block.
Lovastatin :
In a ten-subject study, coadministration of diltiazem (120 mg bid, diltiazem SR) with lovastatin resulted in a 3-4 times increase in mean lovastatin AUC and Cmax versus lovastatin alone; no change in pravastatin AUC and Cmax was observed during diltiazem coadministration. Diltiazem plasma levels were not significantly affected by lovastatin or pravastatin.
Rifampin :
Administration of diltiazem with rifampin markedly reduced plasma diltiazem concentrations and the therapeutic effect of diltiazem.
Short and Long-acting Nitrates :
Diltiazem may be safely coadministesssred with nitrates, but there have been few controlled studies to evaluate the antianginal effectiveness of this combination.
COMPATIBILITY :
DILTIAZEM INJECTION I.P. was tested for compatibility with three commonly used intravenous fluids at a maximal concentration of 1 mg diltiazem hydrochloride per milliliter. DILTIAZEM INJECTION I.P. was found to be physically compatible and chemically stable in the following parenteral solutions for at least 24 hours when stored in glass or polyvinylchloride (PVC) bags at controlled room temperature 15° to 30°C (59° to 86°F) or under refrigeration 2° to 8°C (36° to 46°F).
Dextrose (5 %) injection USP Sodium chloride (0.9 %) injection USP Dextrose (5 %) and sodium chloride (0.45 %) injection USP Because of potential physical incompatibilities, it is recommended that DILTIAZEM INJECTION I.P. not be mixed with any other drugs in the same container. If possible, it is recommended that DILTIAZEM INJECTION I.P. not be co-infused in the same intravenous line.
INCOMPATIBILITIES :
Physical incompatibilities (precipitate formation or cloudiness) were observed when DILTIAZEM INJECTION I.P. was infused in the same intravenous line with the following drugs : acetazolamide, acyclovir, aminophylline, ampicillin, ampicillin sodium/sulbactam sodium, cefamandole, cefoperazone, diazepam, furosemide, hydrocortisone sodium succinate, insulin (regular; 100 units/ml), methylprednisolone sodium succinate, mezlocillin, nafcillin, phenytoin, rifampin, and sodium bicarbonate.
SIDE EFFECTS :
Cardiovascular :
Peripheral oedema; hypotension (especially during initial treatment or with dose increases); bradycardia; angina; AV block; abnormal ECG; arrhythmias.
Central Nervous System :
Dizziness, lightheadedness; headache; weakness; shakiness; somnolence; asthenia.
Dermatologic :
Dermatitis; photosensitivity; petechiae; rash; hair loss; erythema multiforme; Stevens-Johnson syndrome.
Gastrointestinal :
Nausea; vomiting; constipation; abdominal discomfort; cramps; dyspepsia; dry mouth.
Haematologic :
Leucopenia.
Other :
Flushing; micturation disorder; gingival hyperplasia; gynaecomastia; joint pain.
INFORMATION FOR PATIENTS :
- Caution patient to avoid sudden position changes to prevent orthostatic hypotension.
- Notify health care provider if irregular heartbeat, shortness of breath, swelling of the hands and feet, pronounced dizziness, constipation, nausea or hypertension develop.
- If dose is missed, but remembered shortly after it was scheduled, take the missed dose. However, if time is close to next scheduled dose, skip the missed dose.
- Patients are advised to avoid intake of alcoholic beverages and OTC medications without consulting health care provider.
OVERDOSAGE AND TREATMENT OF OVERDOSAGE :
Overdosage experience is limited. In the event of overdosage or an exaggerated response, appropriate supportive measures should be employed. The following measures may be considered :
Bradycardia :
Administer atropine (0.60 to 1.0 mg). If there is no response to vagal blockade administer isoproterenol cautiously.
High-Degree AV Block :
Treat as for bradycardia above. Fixed high-degree AV block should be treated with cardiac pacing.
Cardiac Failure :
Administer inotropic agents (isoproterenol, dopamine, or dobutamine) and diuretics.
Hypotension :
Vasopressors (e.g. dopamine or norepinephrine)
The effectiveness of intravenous calcium administration to reverse the pharmacological effects of diltiazem overdose has been inconsistent. In a few reported cases, overdose with calcium channel blockers associated with hypotension and bradycardia that was initially refractory to atropine became more responsive to atropine after the patients received intravenous calcium. In some cases intravenous calcium has been administered (1 g calcium chloride or 3 g calcium gluconate) over 5 minutes, and repeated every 10-20 minutes as necessary. Calcium gluconate has also been administrated as a continuous infusion at a rate of 2 g per hour for 10 hours. Infusions of calcium for 24 hours or more may be required. Patients should be monitored for signs of hypercalcaemia.
Actual treatment and dosage should depend on the severity of the clinical situation and the judgement and experience of the treating physician. Diltiazem does not appear to be removed by peritoneal or haemodialysis. Limited data suggest that plasmapheresis or charcoal haemoperfusion may hasten diltiazem elimination following overdose. The intravenous LD50’s in mice and rats were 58-61 and 38-39 mg/kg, respectively. The toxic dose in man is not known.
PHARMACEUTICAL PRECAUTIONS :
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
STORAGE :
Store in a refrigerator between 2°C to 8°C (36°F to 46°F), protected from light. Do not freeze.
SHELF LIFE :
24 months from the date of manufacture.
PRESENTATION :
DILTIAZEM INJECTION I.P. is supplied as 25 mg of Diltiazem Hydrochloride USP in 5 ml aqueous solution.
Such 5 ampoules of 5 ml are packed in a box.
Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.

 Cardiovascular
Cardiovascular





