
10 mg, 25 mg, 50 mg
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
PYRIDOXINE TABLETS B.P. (Pyridoxine Hydrochloride) is a nutritional supplement. Chemically, Pyridoxine Hydrochloride is (5-Hydroxy-6-methylpyridine-3,4-diyl)dimethanol hydrochloride. The molecular formula is C8H11NO3,HCl and molecular weight is 205.6.
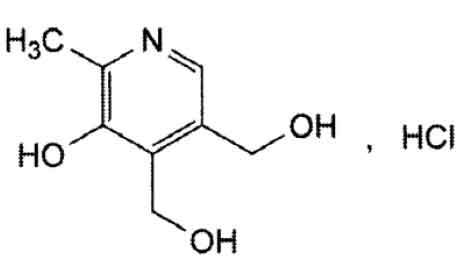
STRUCTURAL FORMULA :
Its structural formula is :
PYRIDOXINE TABLETS B.P. are white coloured, circular, biconvex film coated tablets having breakline on one side and “SGP” embossed on other side.
COMPOSITION :
Each film coated tablet contains :
Pyridoxine Hydrochloride B.P. 25 mg
Excipients q.s.
Colour : Titanium Dioxide B.P.
ACTIONS :
Pyridoxine (vitamin B6) is a water-soluble vitamin involved principally in amino acid metabolism, but is also involved in carbohydrate and fat metabolism. It is also required for the formation of haemoglobin. Pyridoxine deficiency is rare in humans because of its widespread distribution in foods. Pyridoxine deficiency may be drug induced, and inadequate utilization of pyridoxine may result from certain inborn errors of metabolism. Pyridoxine deficiency may lead to sideroblastic anaemia, dermatitis, cheilosis and neurological symptoms such as peripheral neuritis and convulsions.
PHARMACOKINETICS :
Pyridoxine is readily absorbed from the gastrointestinal tract after oral administration and converted to the active forms pyridoxal phosphate and pyridoxamine phosphate. They are stored mainly in the liver where there is metabolisation to 4-pyridoxic acid and other inactive metabolites which are excreted in the urine. As the dose increases proportionally greater amounts are excreted unchanged in the urine. Vitamin B6 crosses the placenta and also appears in breast milk.
INDICATIONS :
PYRIDOXINE TABLETS B.P. are indicated for prevention and management of vitamin B6 deficiency. Treatment of sideroblastic anaemias, homocystinuria or primary hyperoxaluria. Vitamin B6 dependency in infants.
Administration :
PYRIDOXINE TABLETS B.P. are for oral administration only.
Dosage :
In preventing vitamin deficiencies adequate dietary intake is preferred over supplementation whenever possible. An adequate human diet in most circumstances is one containing between 1 and 2 mg vitamin B6 daily. Doses of up to 150 mg daily have been used in general deficiency states. Higher doses of between 200 - 600 mg daily have been used in the treatment of sideroblastic anaemias, with similar doses being used to treat certain metabolic disorders such as homocystinuria or primary hyperoxaluria. Lifelong
supplementation may be required to prevent reoccurrence. Some infants require I.M. or I.V. administration for seizures due to vitamin B6 dependency and some may require lifelong supplementation with oral doses of 2 - 100 mg.
CONTRAINDICATIONS :
Known hypersensitivity to PYRIDOXINE TABLETS B.P. or any of the excipients. PYRIDOXINE TABLETS B.P. contains lactose which is contra-indicated in patients with galactosaemia, the glucose-galactose malabsorption syndrome, or lactase deficiency.
WARNINGS AND PRECAUTIONS :
Vitamin B6 is relatively nontoxic at normal doses however long-term administration of high doses (2 - 6 gm daily) is associated with the development of severe peripheral neuropathies. There have been reports of doses of 500 mg daily having a toxic effect. PYRIDOXINE TABLETS B.P. is presumed to be safe or unlikely to produce an effect on the ability to drive or use machinery. PYRIDOXINE TABLETS B.P. should be used cautiously in diabetic patients.
Pregnancy : Category A
Problems in humans have not been documented with intake of normal daily recommended amounts. However, exposure to large doses of pyridoxine in utero may result in a pyridoxine dependency syndrome in the neonate.
Nursing Mothers :
Problems in humans have not been documented with intake of normal daily recommended amounts.
Paediatric Use :
Problems in humans have not been documented with intake of normal daily recommended amounts.
INTERACTIONS :
Pyridoxine increases the peripheral metabolisation of levodopa. When levodopa is combined with carbidopa this effect is prevented. Isoniazid, cycloserine, pyrazinamide and penicillamine may antagonise the effects of pyridoxine and lead to a secondary deficiency. It has been reported that pyridoxine decreases serum concentrations of phenobarbitone. Patients taking oestrogens e.g. oral contraceptives have higher vitamin B6 requirements.
SIDE EFFECTS :
Nausea, headache, paresthesia, somnolence and low serum folic acid concentrations have been reported. Vitamin B6 is relatively nontoxic at normal doses however long-term administration of high doses (2 - 6 gm daily) is associated with the development of severe peripheral neuropathies. There have been reports of doses of 500 mg daily having a toxic effect. Transient dependency symptoms may occur upon withdrawal of therapy at a dose of 200 mg/day for over 1 month. The significance of this is not known however for patients on large doses for long period of time withdrawal of therapy should probably be gradual.
OVERDOSAGE AND TREATMENT OF OVERDOSAGE :
Sensory neuropathy can occur following long term administration of large doses. Withdrawal should be started but should probably be gradual to prevent the occurrence of transient dependency symptoms.
STORAGE :
Store below 30°C (86°F), protected from moisture and light.
Do not refrigerate.
SHELF LIFE :
24 months from date of manufacture.
PRESENTATION :
PYRIDOXINE TABLETS B.P. contains Pyridoxine Hydrochloride B.P. 25 mg.
500 tablets are packed in bottle.
Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.

 Cardiovascular
Cardiovascular





