10 mg/ml
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
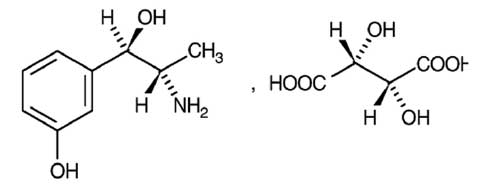
METARAMINOL INJECTION (Metaraminol Tartrate) is a potent sympathomimetic amine that increases both systolic and diastolic blood pressure. Chemically, Metaraminol Tartrate is (1R,2S)-2-amino-1-(3-hydroxyphenyl)propan-1-ol hydrogen (2R,3R)-tartrate. The molecular formula is C9H13NO2,C4H6O6 and molecular weight is 317.3.
STRUCTURAL FORMULA :
Its structural formula is :
METARAMINOL INJECTION is a clear, colourless sterile solution filled in ampoule or vial of suitable size.
COMPOSITION :
Each ml contains :
Metaraminol Tartrate B.P.
equivalent to Metaraminol 10 mg
Methyl Hydroxybenzoate B.P. 0.15 % w/w
Propyl Hydroxybenzoate B.P. 0.02 % w/w
(as preservatives)
Water for Injections B.P. q.s.
ACTIONS :
Acts directly on alpha receptors, causing peripheral vasoconstriction. Increase in systolic, diastolic blood and pulmonary pressure results, as does increase in cardiac output.
PHARMACOKINETICS :
Metaraminol acts about 10 minutes after intramuscular injection with a duration of action of up to about 1 hour. Effects are seen 1 to 2 minutes after intravenous injection with duration of action of about 20 minutes.
INDICATIONS :
METARAMINOL INJECTION is indicated for prevention and treatment of acute hypotensive state occurring with spinal anaesthesia. It is also indicated as adjunctive treatment of hypotension due to haemorrhage, reactions to medications, surgical complications and shock associated with brain damage due to trauma or tumour. Probably effective as adjunct in hypotension due to cardiogenic shock or septicaemia.
Administration : FOR I.M. / I.V. / S.C. USE
INSTRUCTIONS FOR USE OF AMPOULE :
The ampoule used in this product is equipped with O.P.C (One Point Cut) opening system. No ampoule file is needed to open the ampoule. The neck of the ampoule is prescored at the point of constriction. A coloured dot on the ampoule head helps to orientate the ampoule. Take the ampoule and face the coloured dot. Let the solution at the head of the ampoule to flow down by shaking or a gentle stroke. The ampoule opens easily by placing the thumb on the coloured dot and gently pressing downwards as shown.

Dosage :
Usual adult dose :
Hypotension (prophylaxis) :
Intramuscular or subcutaneous, 2 to 10 mg (base).
Hypotension (treatment) :
Intravenous infusion, 15 to 100 mg (base) in 500 ml of 0.9 % sodium chloride injection or 5 % dextrose injection, administered at a rate adjusted to maintain the desired blood pressure.
Shock, severe :
Intravenous, 500 mcg (0.5 mg) to 5 mg (base), followed by an intravenous infusion of metaraminol for control of blood pressure.
Usual adult prescribing limits :
Intravenous infusion, up to 500 mg (base) in 500 ml of infusion fluid (with caution).
Usual paediatric dose :
Dosage has not been established.
CONTRAINDICATIONS :
Use of METARAMINOL INJECTION with cyclopropane or halothane anaesthesia should be avoided, unless clinical circumstances demand such use. Hypersensitivity to any component of this product, including sulfites.
WARNINGS :
Use of sympathomimetic amines with monoamine oxidase inhibitors or tricyclic antidepressants may result in potentiation of the pressor effect. METARAMINOL INJECTION contains sodium bisulfite, a sulfite that may cause allergic-type reactions including anaphylactic symptoms and life-threatening or less severe asthmatic episodes in certain susceptible people. The overall prevalence of sulfite sensitivity in the general population is unknown and probably low. Sulfite sensitivity is seen more frequently in asthmatic than in nonasthmatic people.
PRECAUTIONS :
General :
Caution should be used to avoid excessive blood pressure response. Rapidly induced hypertensive responses have been reported to cause acute pulmonary oedema, arrhythmias, cerebral haemorrhage, or cardiac arrest. Patients with cirrhosis should be treated with caution, with adequate restoration of electrolytes if diuresis ensues. Fatal ventricular arrhythmia was reported in one patient with Laennec’s cirrhosis while receiving metaraminol bitartrate. In several instances, ventricular extrasystoles that appeared during infusion of this vasopressor subsided promptly when the rate of infusion was reduced. With the prolonged action of METARAMINOL INJECTION, a cumulative effect is possible. If there is an excessive vasopressor response there may be a prolonged elevation of blood pressure even after discontinuation of therapy. When vasopressor amines are used for long periods, the resulting vasoconstriction may prevent adequate expansion of circulating volume and may cause perpetuation of shock. There is evidence that plasma volume may be reduced in all types of shock, and that the measurement of central venous pressure is useful in assessing the adequacy of the circulating blood volume. Therefore, blood or plasma volume expanders should be used when the principal reason for hypotension or shock is decreased circulating volume. Because of its vasoconstrictor effect, METARAMINOL INJECTION should be given with caution in heart or thyroid disease, hypertension, or diabetes. Sympathomimetic amines may provoke a relapse in patients with a history of malaria.
Pregnancy : Category C.
Animal reproduction studies have not been conducted with METARAMINOL INJECTION. It is not known whether METARAMINOL INJECTION can cause foetal harm when given to a pregnant woman or can affect reproduction capacity. METARAMINOL INJECTION should be given to a pregnant woman only if clearly needed.
Nursing Mothers :
It is not known whether this drug is secreted in human milk. Because many drugs are secreted in human milk, caution should be exercised when METARAMINOL INJECTION is given to a nursing woman.
Paediatric Use :
Safety and effectiveness in paediatric patients have not been established.
INTERACTIONS AND INCOMPATIBILITIES :
Guanethidine : Antihypertensive effects of guanethidine may be negated.
MAO inhibitors, furazolidone, rauwolfia alkaloids, methyldopa :
May significantly increase pressor response, possibly resulting in hypertensive crisis and intracranial haemorrhage.
Tricyclic antidepressants :
May decrease pressor response.
Incompatibilities :
Metaraminol bitartrate tends to be physically incompatible with other medications of poor solubility in acidic media, such as sodium salts of barbiturates, penicillins & phenytoin.
SIDE EFFECTS :
Sympathomimetic amines, including METARAMINOL INJECTION, may cause sinus or ventricular tachycardia, or other arrhythmias, especially in patients with myocardial infarction. In patients with a history of malaria, these compounds may provoke a relapse. Abscess formation, tissue necrosis, or sloughing rarely may follow the use of METARAMINOL INJECTION. In choosing the site of injection, it is important to avoid those areas recognized as not suitable for use of any pressor agent and to discontinue the infusion immediately if infiltration or thrombosis occurs. Although the physician may be forced by the urgent nature of the patient’s condition to choose injection sites that are not recognized as suitable, he should, when possible, use the preferred areas of injection. The larger veins of the antecubital fossa or the thigh are preferred to veins in the dorsum of the hand or ankle veins, particularly in patients with peripheral vascular disease, diabetes mellitus, Buerger’s disease, or conditions with coexistent.
INFORMATION FOR PATIENTS :
Alert patient to the possible problems of extravasation. Instruct patient to report symptoms such as paresthesia, respiratory distress, chest pain, palpitations or irritation or pain at injection site. Educate patient and family regarding sympathomimetic side effects.
OVERDOSAGE :
Overdosage may result in severe hypertension accompanied by headache, constricting sensation in the chest, nausea, vomiting, euphoria, diaphoresis, pulmonary oedema, tachycardia, bradycardia, sinus arrhythmia, atrial or ventricular arrhythmias, cerebral haemorrhage, myocardial infarction, cardiac arrest or convulsions. The oral LD50 in the rat and mouse is 240 mg/kg and 99 mg/kg, respectively.
TREATMENT OF OVERDOSAGE :
Should an excessive elevation of blood pressure occur, it may be immediately relieved by a sympatholytic agent, e.g., phentolamine. An appropriate antiarrhythmic agent may also be required.
PHARMACEUTICAL PRECAUTIONS :
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
STORAGE :
Store below 30°C, protected from light.
Do not refrigerate.
SHELF LIFE :
24 months from the date of manufacture.
PRESENTATION :
METARAMINOL INJECTION is supplied as 10 mg/ml Metaraminol in 1 ml and 10 ml aqueous solution.
| Strength | Pack SIze | Package |
| 10 mg/ml (base) | 1 ml Ampoule | 10 Ampoules |
| 10 mg/ml (base) | 10 ml Vial | Single Pack Vial |

 Cardiovascular
Cardiovascular