
10 mg/ml
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
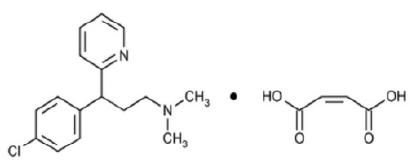
CHLORPHENIRAMINE MALEATE INJECTION USP (Chlorpheniramine Maleate) is an alkylamine derivative, sedating antihistamine that causes a moderate degree of sedation; it also has antimuscarinic activity. Chemically, Chlorpheniramine Maleate is 2-[p-Chloro-α-[2-dimethylamino)ethyl]benzyl]pyridine maleate (1:1). The molecular formula is C16H19ClN2·C4H4O4 and molecular weight is 390.86.
STRUCTURAL FORMULA :
Its structural formula is :
CHLORPHENIRAMINE MALEATE INJECTION USP is a sterile, clear, colourless aqueous solution filled in amber ampoule of suitable size.
COMPOSITION :
Each ml contains :
Chlorpheniramine Maleate USP 10 mg
Chlorobutanol USP 0.5 % w/v
(anhydrous)
(As preservative)
Sodium Chloride USP 9 mg
Water for Injection USP q.s.
ACTIONS :
Chlorpheniramine maleate is an alkylamine derivative and potent, long acting H1-receptor antagonist (an antihistamine). Chlorpheniramine maleate diminishes the main actions of histamine in the body by competitive, reversible blockade of histamine receptor sites on tissues. Vasodilatation increased capillary permeability, flare and itch reactions in the skin are blocked by chlorpheniramine maleate. H1-antagonists also possess anticholingeric serotonin- antagonising and local anaesthetic effects.
PHARMACOKINETICS :
Following intravenous administration, the apparent steady-state volume of distribution of chlorpheniramine is approximately 3 L/kg in adults and 3.8 L/kg in children. Chlorpheniramine is approximately 70 % bound to plasma proteins. In adults with normal renal and hepatic function, the terminal elimination half-life of chlorpheniramine reportedly ranges from 12 to 43 hours. The systemic exposure per mg dose is lower in children than adults and the elimination half-life may be shorter. Chlorpheniramine maleate is extensively metabolised. Metabolites include desmethyl- and didesmethylchlorphenamine. Unchanged drug and metabolites are excreted primarily in the urine; excretion is dependent on urinary pH and flow rate. Only trace amounts have been found in the faeces. A duration of action of 4 to 6 hours has been reported; this is shorter than may be predicted from pharmacokinetic parameters.
INDICATIONS :
CHLORPHENIRAMINE MALEATE INJECTION USP is indicated for acute urticaria, control of allergic reactions to insect bites and stings, angioneurotic oedema, drug and serum reactions, desensitisation reactions, hayfever, vasomotor rhinitis, severe pruritus of non-specific origin. CHLORPHENIRAMINE MALEATE INJECTION USP is also effective in relieving the symptoms of seasonal and perennial allergic rhinitis such as sneezing, nasal itch and conjunctivitis. CHLORPHENIRAMINE MALEATE INJECTION USP is also used as an adjunct in the emergency treatment of anaphylactic shock.
Administration :
CHLORPHENIRAMINE MALEATE INJECTION USP is given by the subcutaneous, intramuscular or slow intravenous injection.
INSTRUCTIONS FOR USE OF AMPOULE :
The ampoule used in this product is equipped with O.P.C. (One Point Cut) opening system. No ampoule file is needed to open the ampoule. The neck of the ampoule is prescored at the point of constriction. A coloured dot on the ampoule head helps to orientate the ampoule. Take the ampoule and face the coloured dot. Let the solution at the head of the ampoule to flow down by shaking or a gentle stroke. The ampoule opens easily by placing the thumb on the coloured dot and gently pressing downwards as shown.

Dosage :
Adults :
Chlorpheniramine maleate may be given by intramuscular, by subcutaneous, or by slow intravenous injection over a period of 1 minute. The usual dose is 10 to 20 mg and the total dose given by these routes in 24 hours should not normally exceed 40 mg. When a rapid effect is desired, as in anaphylactic reactions, the intravenous route is recommended in addition to emergency therapy with adrenaline (epinephrine), corticosteroids, oxygen and supportive therapy as required.
Children :
For children, doses of 87.5 micrograms/kg subcutaneously four times daily have been suggested. The following parenteral doses should be calculated based on their age or their body weight :
| Age | Dose |
| 1 Month to 1 Year | 250 micrograms/kg |
| 1 Month to 5 Year | 2.5 to 5 mg |
| 6 Month to 12 Year | 5 to 10 mg |
| 1 Month to 18 Year | 200 micrograms/kg |
The doses for children may be repeated if necessary up to 4 times in 24 hours have been suggested. Extra care should be taken when preparing the injection for children under 1 year due to the small volumes that are required. Dilution of CHLORPHENIRAMINE
MALEATE INJECTION USP with sodium chloride intravenous infusion (0.9 % w/v) should facilitate preparation. For example, diluting 0.2 ml CHLORPHENIRAMINE MALEATE INJECTION USP to 2 ml with sodium chloride 0.9 % injection produces a solution containing chlorpheniramine 1 mg/ml. The diluted product should be used immediately.
CONTRAINDICATIONS :
CHLORPHENIRAMINE MALEATE INJECTION USP is contraindicated in patients who are hypersensitive to antihistamines. CHLORPHENIRAMINE MALEATE INJECTION USP is also contraindicated in patients narrow-angle glaucoma; stenosing peptic ulcer; symptomatic prostatic hypertrophy; asthmatic attack; bladder neck obstruction; pyloroduodenal obstruction; MAO therapy. CHLORPHENIRAMINE MALEATE INJECTION USP is also contraindicated in newborn or premature infants and in nursing mothers.
Pregnancy : Category B
There is inadequate evidence of safety in human pregnancy. CHLORPHENIRAMINE MALEATE INJECTION USP should only be used during pregnancy when clearly needed and when the potential benefits outweigh the potential unknown risks to the foetus. Use during the third trimester may result in reactions in neonates.
Nursing mothers :
It is reasonable to assume that chlorpheniramine may inhibit lactation and may be secreted in breast milk. The use of CHLORPHENIRAMINE MALEATE INJECTION USP in mothers breast-feeding their babies requires that the therapeutic benefits of the drug should be weighed against the potential hazards to the mother and baby.
Paediatric use :
Use of antihistamines is not recommended in newborn or premature infants. This age group may be at a higher risk than other age groups because of an increased susceptibility to anticholinergic effects, such as CNS excitation, and an increased tendency toward convulsions.
EFFECTS ON ABILITY TO DRIVE AND USE MACHINES :
The anticholinergic properties of chlorpheniramine may cause drowsiness, blurred vision and psychomotor impairment, which can seriously hamper the patient’s ability to drive and use machinery.
INTERACTIONS AND INCOMPATIBILITIES :
Administration of CHLORPHENIRAMINE MALEATE INJECTION USP with other CNS depressants such as alcohol, barbiturates, hypnotics, opioid analgesics, sedatives and antipsychotics may enhance sedation. CHLORPHENIRAMINE MALEATE INJECTION USP will enhance the antimuscarinic action of atropine, tricyclic antidepressants and Monoamine Oxidase inhibitors (MAOIs). There is evidence to suggest that antihistamines such as chlorpheniramine maleate could mask the warning signs of damage caused by ototoxic medicines such as aminoglycosides. CHLORPHENIRAMINE MALEATE INJECTION USP inhibits phenytoin metabolism and can lead to phenytoin toxicity. CHLORPHENIRAMINE MALEATE INJECTION USP may suppress the cutaneous histamine response to allergen extracts. Its use should be stopped several days before skin testing.
SIDE EFFECTS :
Cardiovascular :
Orthostatic hypotension; palpitations; bradycardia; tachycardia; reflex tachycardia; extrasystoles; faintness.
Central Nervous System :
Drowsiness (often transient); sedation; dizziness; faintness; disturbed coordination; nervousness; restlessness.
Gastrointestinal :
Dry mouth; epigastric distress; anorexia; nausea; vomiting; diarrhoea; constipation; change in bowel habits.
Genitourinary :
Urinary frequency or retention; dysuria.
Haematologic :
Haemolytic anaemia; thrombocytopenia; agranulocytosis.
Metabolic :
Increased appetite; weight gain.
Respiratory :
Thickening of bronchial secretions; chest tightness; wheezing; nasal stuffiness; dry nose and throat; sore throat; respiratory depression.
Other :
Hypersensitivity reactions; photosensitivity.
OVERDOSAGE :
Symptoms :
The estimated lethal dose of chlorpheniramine is 25 mg to 50 mg/kg body weight. Symptoms and signs include sedation, paradoxical stimulation of the CNS, toxic psychosis, seizures, apnoea, convulsions, anticholinergic effects, dystonic reactions and cardiovascular collapse including arrhythmias.
TREATMENT OF OVERDOSAGE :
Symptomatic and supportive measures should be provided with special attention to cardiac, respiratory, renal and hepatic functions, and fluid and electrolytic balance. If overdosage is by the oral route, treatment should include gastric lavage or induced emesis using syrup of ipecacuanha. Following these measures activated charcoal and cathartics may be administered to minimise absorption. Treat hypotension and arrhythmias vigorously. CNS convulsions may be treated with intravenous diazepam. Haemoperfusion may be used in severe cases.
PHARMACEUTICAL PRECAUTIONS :
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
STORAGE :
Store below 30°C (86°F), protected from light. Do not refrigerate.
SHELF LIFE :
24 months from the date of manufacture.
PRESENTATION :
CHLORPHENIRAMINE MALEATE INJECTION USP is supplied as 10 mg of Chlorpheniramine Maleate USP in 1 ml aqueous solution.
Such 10 ampoules of 1 ml are packed in a Box.
Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.

 Cardiovascular
Cardiovascular





