
250mg
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
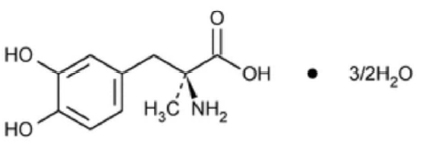
METHYLDOPA TABLETS USP (Methyldopa) is an antihypertensive drug. Chemically, Methyldopa is L-Tyrosine, 3-hydroxy-α-methyl-, sesquihydrate. The molecular formula is C10H13NO4 ·1½ H2O and molecular weight is 238.24.
STRUCTURAL FORMULA :
Its structural formula is :
METHYLDOPA TABLETS USP is yellow coloured, circular, biconvex film coated tablet.
COMPOSITION :
Each film coated tablet contains :
Methyldopa (anhydrous) USP 250 mg
Excipients q.s.
Colours : Aluminium Lake Sunset Yellow, Tartrazine,
Titanium Dioxide USP
ACTIONS :
Methyldopa is an antihypertensive that is thought to have a mainly central action. It is decarboxylated in the CNS to alpha-methylnoradrenaline, which is thought to stimulate alpha adrenoceptors resulting in a reduction in sympathetic tone and a fall in blood pressure. It may also act as a false neurotransmitter, and have some inhibitory actions on plasma renin activity. Methyldopa reduces the tissue concentrations of dopamine, noradrenaline, adrenaline, and serotonin.
PHARMACOKINETICS :
After oral use methyldopa is variably and incompletely absorbed, apparently by an amino-acid active transport system. The mean bioavailability has been reported to be about 50 %. It is extensively metabolised and is excreted in urine mainly as unchanged drug and the O-sulfate conjugate. It crosses the blood-brain barrier and is decarboxylated in the CNS to active alpha-methyl-noradrenaline. The elimination is biphasic with a half-life of about 1.7 hours in the initial phase; the second phase is more prolonged. Clearance is decreased and half-life prolonged in renal impairment. Plasma protein binding is reported to be minimal. Methyldopa crosses the placenta; small amounts are distributed into breast milk.
INDICATIONS :
METHYLDOPA TABLETS USP is indicated for the treatment of hypertension (mild, moderate or severe).
Administration :
METHYLDOPA TABLETS USP is for oral administration.
Dosage :
General :
Methyldopa is largely excreted by the kidney and patients with impaired renal function may respond to smaller doses. Syncope in older patients may be related to an increased sensitivity and advanced arteriosclerotic vascular disease. This may be avoided by lower doses. Withdrawal of methyldopa is followed by return of hypertension usually within 48 hours. This is not complicated by an overshoot of blood pressure. Therapy with methyldopa may be initiated in most patients already on treatment with other antihypertensive agents.
Methyldopa may also be used concomitantly with amiloride/hydrochlorothiazide tablets or beta-blocking agents, such as timolol maleate. Many patients can be controlled with one tablet of amiloride 5 mg/hydrochlorothiazide 50 mg and 500 mg of methyldopa administered once daily. When methyldopa is given to patients on other antihypertensives, the dose of these agents may need to be adjusted to effect a smooth transition. Terminate these antihypertensive medications gradually if required (see manufacturers’ recommendations on stopping these medicines). Following such previous antihypertensive therapy, the initial dose of methyldopa should be limited to not more than 500 mg daily and increased as required at intervals of not less than 2 days.
Adults :
The usual starting dosage of methyldopa is 250 mg two or three times a day in the first 48 hours. The daily dosage then may be increased or decreased, preferably at intervals of not less than two days, until an adequate response is achieved. The maximum recommended daily dosage is 3 gm. When methyldopa 500 mg is added to 50 mg of hydrochlorothiazide, the two agents may be given together once daily. Many patients experience sedation for two or three days when therapy with methyldopa is started or when the dose is increased. When increasing the dosage, therefore, it may be desirable to increase the evening dose first.
Children :
Initial dosage is based on 10 mg/kg of body weight daily in two or four doses. The daily dosage then is increased or decreased until an adequate response is achieved. The maximum dosage is 65 mg/kg or 3.0 gm daily, whichever is less.
CONTRAINDICATIONS :
METHYLDOPA TABLETS USP is contra-indicated in patients :
- with active hepatic disease, such as acute hepatitis and active cirrhosis.
- with hypersensitivity to the active substance (including hepatic disorders associated with previous methyldopa therapy) or to any of the excipients.
- with depression.
- on therapy with monoamine oxidase inhibitors (MAOIs).
- with a catecholamine-secreting tumour such as phaeochromocytoma or paraganglioma.
- with porphyria.
METHYLDOPA TABLETS USP contains lactose which is contra-indicated in patients with galactosaemia, the glucose-galactose malabsorption syndrome, or lactase deficiency.
It is important to recognize that a positive Coombs test, haemolytic anaemia, and liver disorders may occur with methyldopa therapy. The rare occurrences of haemolytic anaemia or liver disorders could lead to potentially fatal complications unless properly recognized and managed. With prolonged methyldopa therapy, 10 to 20 percent of patients develop a positive direct Coombs test which usually occurs between 6 and 12 months of methyldopa therapy. Lowest incidence is at daily dosage of 1 gm or less. This on rare occasions may be associated with haemolytic anaemia, which could lead to potentially fatal complications. One cannot predict which patients with a positive direct Coombs test may develop haemolytic anaemia. Prior existence or development of a positive direct Coombs test is not in itself a contraindication to use of methyldopa. If a positive Coombs test develops during methyldopa therapy, the physician should determine whether haemolytic anaemia exists and whether the positive Coombs test may be a problem. For example, in addition to a positive direct Coombs test there is less often a positive indirect Coombs test which may interfere with cross matching of blood.
Before treatment is started, it is desirable to do a blood count (haematocrit, haemoglobin, or red cell count) for a baseline or to establish whether there is anaemia. Periodic blood counts should be done during therapy to detect haemolytic anaemia. It may be useful to do a direct Coombs test before therapy and at 6 and 12 months after the start of therapy. If Coombs-positive haemolytic anaemia occurs, the cause may be methyldopa and the drug should be discontinued. Usually the anaemia remits promptly. If not, corticosteroids may be given and other causes of anaemia should be considered. If the haemolytic anaemia is related to methyldopa, the drug should not be reinstituted. When methyldopa causes Coombs positivity alone or with haemolytic anaemia, the red cell is usually coated with gamma globulin of the lgG (gamma G) class only. The positive Coombs test may not revert to normal until weeks to months after methyldopa is stopped. Should the need for transfusion arise in a patient receiving methyldopa, both a direct and an indirect Coombs test should be performed. In the absence of haemolytic anaemia, usually only the direct Coombs test will be positive. A positive direct Coombs test alone will not interfere with typing or cross matching. If the indirect Coombs test is also positive, problems may arise in the major cross match and the assistance of a haematologist or transfusion expert will be needed.
Occasionally, fever has occurred within the first three weeks of methyldopa therapy, associated in some cases with eosinophilia or abnormalities in one or more liver function tests, such as serum alkaline phosphatase, serum transaminases (SGOT, SGPT), bilirubin, and prothrombin time. Jaundice, with or without fever, may occur with onset usually within the first two to three months of therapy. In some patients the findings are consistent with those of cholestasis. In others the findings are consistent with hepatitis and hepatocellular injury.
Rarely, fatal hepatic necrosis has been reported after use of methyldopa. These hepatic changes may represent hypersensitivity reactions. Periodic determinations of hepatic function should be done particularly during the first 6 to 12 weeks of therapy or whenever an unexplained fever occurs. If fever, abnormalities in liver function tests, or jaundice appear, stop therapy with methyldopa. If caused by methyldopa, the temperature and abnormalities in liver function characteristically have reverted to normal when the drug was discontinued. Methyldopa should not be reinstituted in such patients. Rarely, a reversible reduction of the white blood cell count with a primary effect on the granulocytes has been seen. The granulocyte count returned promptly to normal on discontinuance of the drug. Rare cases of granulocytopenia have been reported. In each instance, upon stopping the drug, the white cell count returned to normal. Reversible thrombocytopenia has occurred rarely. The tablet contains “tartrazine” colour which may cause allergic reactions, including bronchial asthma, especially in patients who are allergic to the acetyl salicylic acid.
PRECAUTIONS :
General :
Methyldopa should be used with caution in patients with a history of previous liver disease or Dysfunction. Some patients taking methyldopa experience clinical oedema or weight gain which may be controlled by use of a diuretic. Methyldopa should not be continued if oedema progresses or signs of heart failure appear. Hypertension has recurred occasionally after dialysis in patients given methyldopa because the drug is removed by this procedure. Rarely, involuntary choreoathetotic movements have been observed during therapy with methyldopa in patients with severe bilateral cerebrovascular disease. Should these movements occur, stop therapy. METHYLDOPA TABLETS USP should be used cautiously in diabetic patients.
Laboratory Tests :
Blood count, Coombs test and liver function tests are recommended before initiating therapy and at periodic intervals.
Pregnancy : Category B
Methyldopa has been used under close medical and obstetric supervision for the treatment of hypertension during pregnancy. There was no clinical evidence that methyldopa caused foetal abnormalities or affected the neonate. Published reports of the use of methyldopa during all trimesters indicate that if this drug is used during pregnancy the possibility of foetal harm appears remote. In clinical studies, treatment with methyldopa has been associated with an improved foetal outcome. The majority of the women in these studies were in the third trimester when methyldopa therapy was begun. Methyldopa does cross the placental barrier and appears in cord blood. Although no obvious teratogenic effects have been reported, the possibility of foetal injury cannot be excluded and the use of the medicine in women who are or may become pregnant requires that anticipated benefits be weighed against possible risks.
Nursing mothers :
Methyldopa appears in breast milk. Therefore, caution should be exercised when methyldopa is given to a nursing woman.
Paediatric Use :
There are no well-controlled clinical trials in paediatric patients. Information on dosing in paediatric patients is supported by evidence from published literature regarding the treatment of hypertension in paediatric patients.
INTERACTIONS AND INCOMPATIBILITIES :
Lithium :
When methyldopa and lithium are given concomitantly the patient should be monitored carefully for symptoms of lithium toxicity.
Other antihypertensive drugs :
When methyldopa is used with other antihypertensive drugs, potentiation of antihypertensive action may occur. The progress of patients should be carefully followed to detect side reactions or manifestations of drug idiosyncrasy.
Other classes of drug :
The antihypertensive effect of METHYLDOPA TABLETS USP may be diminished by sympathomimetics, phenothiazines, tricyclic antidepressants and monoamine oxidase inhibitors. In addition, phenothiazines may have additive hypotensive effects.
Iron :
Several studies demonstrate a decrease in the bioavailability of methyldopa when it is ingested with ferrous sulphate or ferrous gluconate. This may adversely affect blood pressure control in patients treated with methyldopa.
Interference with laboratory tests :
Methyldopa may interfere with the measurement of urinary uric acid by the phosphotungstate method, serum creatinine by the alkaline picrate method, and AST (SGOT) by colorimetric method. Interference with spectrophotometric methods for AST (SGOT) analysis has not been reported. As methyldopa fluoresces at the same wavelengths as catecholamines, spuriously high amounts of urinary catecholamines may be reported interfering with a diagnosis of phaeochromocytoma. It is important to recognise this phenomenon before a patient with a possible phaeochromocytoma is subjected to surgery. Methyldopa does not interfere with measurements of VMA (vanillylmandelic acid) by those methods which convert VMA to vanillin. Rarely, when urine is exposed to air after voiding, it may darken because of breakdown of methyldopa or its metabolites.
SIDE EFFECTS :
The following reactions have been reported :
Cardiac disorders :
Bradycardia, aggravation of angina pectoris, myocarditis, pericarditis.
Blood and lymphatic system disorders :
Haemolytic anaemia, bone-marrow depression, leucopenia, granulocytopenia, thrombocytopenia, eosinophilia.
Nervous system disorders :
Sedation (usually transient), headache, paraesthesia, Parkinsonism, Bell’s palsy, involuntary choreoathetotic movements. Impaired mental acuity, prolonged carotid sinus hypersensitivity. Dizziness, light-headedness, and symptoms of cerebrovascular insufficiency (may be due to lowering of blood pressure).
Respiratory, thoracic and mediastinal disorders :
Nasal stuffiness.
Gastrointestinal disorders :
Nausea, vomiting, distension, constipation, flatus, diarrhoea, colitis, mild dryness of mouth, sore or ‘black’ tongue, pancreatitis.
Skin and subcutaneous tissue disorders :
Rash as in eczema or lichenoid eruption, toxic epidermal necrolysis.
Musculoskeletal and connective tissue disorders :
Lupus-like syndrome, mild arthralgia with or without joint swelling, myalgia.
Endocrine disorders :
Hyperprolactinaemia.
Infections and infestations :
Sialadenitis.
Vascular disorders :
Orthostatic hypotension (decrease daily dosage).
General disorders and administrative site conditions :
Asthenia or weakness, oedema (and weight gain) usually relieved by use of a diuretic. (Discontinue methyldopa if oedema progresses or signs of heart failure appear.), drug-related fever.
Hepatobiliary disorders :
Liver disorders including hepatitis, jaundice.
Reproductive system and breast disorders :
Breast enlargement, gynaecomastia, amenorrhoea, lactation, impotence, failure of ejaculation.
Psychiatric disorders :
Psychic disturbances including nightmares, reversible mild psychoses or depression, decreased libido.
Investigations :
Positive Coombs test, positive tests for antinuclear antibody, LE cells, and rheumatoid factor, abnormal liver-function tests, rise in blood urea.
EFFECTS ON ABILITY TO DRIVE AND USE MACHINES :
METHYLDOPA TABLETS USP may cause sedation, usually transient, during the initial period of therapy or whenever the dose is increased. If affected, patients should not carry out activities where alertness is necessary, such as driving a car or operating machinery.
OVERDOSAGE :
Symptoms :
Acute overdosage may produce acute hypotension with other responses attributable to brain and gastro-intestinal malfunction (excessive sedation, weakness, bradycardia, dizziness, light-headedness, constipation, distension, flatus, diarrhoea, nausea, and vomiting).
TREATMENT OF OVERDOSAGE :
If ingestion is recent, emesis may be induced or gastric lavage performed. There is no specific antidote. Methyldopa is dialysable. Treatment is symptomatic. Infusions may be helpful to promote urinary excretion. Special attention should be directed towards cardiac rate and output, blood volume, electrolyte balance, paralytic ileus, urinary function and cerebral activity. Administration of sympathomimetic agents may be indicated. When chronic overdosage is suspected, METHYLDOPA TABLETS USP should be discontinued.
STORAGE :
Store below 30°C (86°F), protected from moisture and light.
Do not refrigerate.
SHELF LIFE :
24 months from the date of manufacture.
PRESENTATION :
METHYLDOPA TABLETS USP contains Methyldopa (anhydrous) USP 250 mg.
1 Blister of 10 Tablets per Box.
Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.

 Cardiovascular
Cardiovascular





