
20 mg/2 ml
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
FUROSEMIDE INJECTION USP (Furosemide) is a potent diuretic with rapid action which is an anthranilic acid derivative. Chemically, Furosemide is 4-chloro-N-furfuryl-5-sulfamoylanthranilic acid. The molecular formula is C12H11CIN2O5S and molecular weight is 330.74.
STRUCTURAL FORMULA :
Its structural formula is :
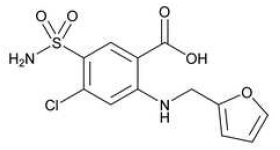
FUROSEMIDE INJECTION USP is a sterile clear, colourless solution filled in amber ampoule of suitable size.
COMPOSITION :
Each ml contains :
Furosemide USP 10 mg
Sodium Chloride USP 7.5 mg
Water for Injection USP q.s.
Contains no preservatives.
ACTIONS :
Furosemide is a potent diuretic with a rapid action. It inhibits sodium and chloride absorption in the ascending limb of Henle’s loop and in both the proximal and distal tubules. The high degree of efficacy is due to this unique site of action. The action on the distal tubule is independent of any inhibitory effect on carbonic anhydrase or aldosterone. Furosemide may promote diuresis in cases which have previously proved resistant to other diuretics. Furosemide has no significant pharmacological effects other than on renal function.
PHARMACOKINETICS :
Absorption :Furosemide is rapidly absorbed from the gastrointestinal tract. Absorption rates in healthy subjects have been reported from 60-69 % and from 43-46 % in patients with end stage renal failure. The onset of diuresis following intravenous administration is within 5 minutes and somewhat later after intramuscular administration. The peak effect occurs within the first half hour. The duration of diuretic effect is approximately 2 hours.
Distribution :
Furosemide is extensively bound to plasma proteins, mainly to albumin. Plasma concentrations ranging from 1 to 400 μg/ml are 91 to 99 % bound in healthy individuals. The unbound fraction averages 2.3 to 4.1 % at therapeutic concentrations.
Metabolism :
Recent evidence suggests that furosemide glucuronide is the only, or at least the major, biotransformation product of furosemide in man.
Excretion :
In patients with normal renal function, approximately 80 % of an intravenous or intramuscular dose is excreted in the urine within 24 hours. Urinary excretion is accomplished both by glomerular filtration and proximal tubular secretion, which accounts for roughly 66 % of the ingested dose, the remainder being excreted in the faeces. A small fraction is metabolised by cleavage of the side chain.
Half Life :
Furosemide has a biphasic half life in the plasma with T1/2 ranging up to 100 minutes. T1/2 is prolonged by renal and hepatic insufficiency and in premature and full term infants.
INDICATIONS :
Parenteral therapy should be reserved for patients unable to take oral medication or for patients in emergency clinical situations.
Oedema :
FUROSEMIDE INJECTION USP is indicated in adults and paediatric patients for the treatment of oedema associated with congestive heart failure, cirrhosis of the liver, and renal disease, including the nephrotic syndrome. FUROSEMIDE INJECTION USP is particularly useful when an agent with greater diuretic potential is desired. FUROSEMIDE INJECTION USP is indicated as adjunctive therapy in acute pulmonary oedema. The intravenous administration of furosemide is indicated when a rapid onset of diuresis is desired, e.g., in acute pulmonary oedema. If gastrointestinal absorption is impaired or oral medication is not practical for any reason, FUROSEMIDE INJECTION USP is indicated by the intravenous or intramuscular route. Parenteral use should be replaced with oral furosemide as soon as practical.
Administration :
FUROSEMIDE INJECTION USP is given by intramuscular injection or by slow intravenous injection or by slow intravenous infusion. Parenteral therapy with FUROSEMIDE INJECTION USP should be used only in patients unable to take oral medication or in emergency situations and should be replaced with oral therapy as soon as practical.
INSTRUCTIONS FOR USE OF AMPOULE :
The ampoule used in this product is equipped with O.P.C. (One Point Cut) opening system. No ampoule file is needed to open the ampoule. The neck of the ampoule is prescored at the point of constriction. A coloured dot on the ampoule head helps to orientate the ampoule. Take the ampoule and face the coloured dot. Let the solution at the head of the ampoule to flow down by shaking or a gentle stroke. The ampoule opens easily by placing the thumb on the coloured dot and gently pressing downwards as shown.

Dosage :
Oedema :
Adults :
The usual initial dose of FUROSEMIDE INJECTION USP is 20 to 40 mg given as a single dose, injected intramuscularly or intravenously. The intravenous dose should be given slowly. Ordinarily a prompt diuresis ensues. If needed, another dose may be administered in the same manner 2 hours later, or the dose may be increased. The dose may be raised by 20 mg, and given not sooner than 2 hours after the previous dose, until the desired diuretic effect has been obtained. This individually determined single dose should then be given once or twice daily. Therapy should be individualised according to patient response to gain maximal therapeutic response and to determine the minimal dose needed to maintain that response. Close medical supervision is necessary. If the physician elects to use FUROSEMIDE INJECTION USP parenteral therapy, add FUROSEMIDE INJECTION USP to either Sodium Chloride Injection, Lactated Ringer’s Injection, or Dextrose (5 %) Injection after pH has been adjusted to above 5.5. Administer as a controlled intravenous infusion at a rate not greater than 4 mg/min. FUROSEMIDE INJECTION USP is a buffered alkaline solution.
Acute Pulmonary Oedema :
The usual initial dose of FUROSEMIDE INJECTION USP is 40 mg injected slowly intravenously. If a satisfactory response does not occur within 1 hour, the dose may be increased to 80 mg injected slowly intravenously. If necessary, additional therapy (e.g. digitalis,
oxygen) may be administered concomitantly.
Cerebral Oedema :
The following procedure is recommended, pending further
experience :
Intravenous injection of 20 to 40 mg three times daily. A more uniform diuretic action is obtained if the same doses are infused. The rate of infusion must be determined individually in accordance with the diuretic action and the neurological findings. Infants and children : Parenteral therapy should only be used in patients unable to take oral medication or in emergency situations, and should be replaced with oral therapy as soon as practical. The recommended initial dose of FUROSEMIDE INJECTION USP (intravenously or intramuscularly) in infants and children is 1 mg/kg body weight and should be given slowly under close medical supervision. If the diuretic response to the initial dose is not satisfactory, dosage may be increased by 1 mg/kg not sooner than 2 hours after the previous dose, until the desired diuretic effect has been obtained. Doses greater than 6 mg/kg body weight are not recommended.
Use in the elderly :
No requirement exists for special dosage recommendations in the elderly.
CONTRAINDICATIONS :
Known hypersensitivity to furosemide or sulphonamides or any of the inactive ingredients. Patients allergic to sulphonamides (e.g. sulfonamide antibiotics or sulfonylureas) may show cross sensitivity to furosemide. Complete renal shutdown. If increasing azotaemia and oliguria occur during treatment of severe progressive renal disease, discontinue furosemide. Severe hypokalaemia, hyponatraemia, hypovolaemia or hypotension must be regarded as contraindications until serum electrolytes, fluid balance and blood pressure have been restored to normal levels. In hepatic coma or pre-coma and conditions producing electrolyte depletion, furosemide therapy should not be instituted until the underlying conditions have been corrected or ameliorated. In breast feeding women. Do not administer furosemide to newborns presenting jaundice or to infants with conditions which might induce hyperbilirubinaemia or kernicterus (e.g. Rhesus incompatibility, familial non-haemolytic jaundice etc.) because of furosemide’s ‘in vitro’ potential to displace bilirubin from albumin.
In patients with hepatic cirrhosis and ascites, furosemide therapy is best initiated in the hospital. In hepatic coma and in states of electrolyte depletion, therapy should not be instituted until the basic condition is improved. Sudden alterations of fluid and electrolyte balance in patients with cirrhosis may precipitate hepatic coma; therefore, strict observation is necessary during the period of diuresis. Supplemental potassium chloride and, if required, an aldosterone antagonist are helpful in preventing hypokalaemia and metabolic alkalosis. If increasing azotaemia and oliguria occur during treatment of severe progressive renal disease, furosemide should be discontinued. Cases of tinnitus and reversible or irreversible hearing impairment have been reported. Reports usually, indicate that furosemide ototoxicity is associated with rapid injection, severe renal impairment, the use of higher than recommended doses, hypoproteinaemia or concomitant therapy with aminoglycoside antibiotics, ethacrynic acid, or other ototoxic drugs. If the physician elects to use high dose parenteral therapy, controlled intravenous infusion is advisable (for adults, an infusion rate not exceeding 4 mg furosemide per minute has been used).
Paediatric Use :
In premature neonates with respiratory distress syndrome, diuretic treatment with furosemide in the first few weeks of life may increase the risk of persistent patent ductus arteriosus (PDA), possibly through a prostaglandin-E-mediated process. Literature reports indicate that premature infants with post conceptual age (gestational plus postnatal) less than 31 weeks receiving doses exceeding 1 mg/kg/24 hours may develop plasma levels which could be associated with potential toxic effects including ototoxicity. Hearing loss in neonates has been associated with the use of FUROSEMIDE INJECTION USP. In children, urge to defecate, complaints of abdominal pain and cramping have been reported after intravenous furosemide. An association of these symptoms with a low serum calcium and/or a low calcium/protein ratio is possible.
PRECAUTION :
Excessive diuresis may cause dehydration and blood volume reduction with circulatory collapse and possible vascular thrombosis and embolism, particularly in elderly patients. As with any effective diuretic, electrolyte depletion may occur during furosemide therapy, especially in patients receiving higher doses and restricted salt intake. Hypokalaemia may develop with furosemide, especially with brisk diuresis, inadequate oral electrolyte intake, when cirrhosis is present, or during concomitant use of corticosteroids or ACTH, licorice in large amounts or prolong use of laxative. Digitalis therapy may exaggerate metabolic effects of hypokalaemia, especially myocardial effects. All patients receiving furosemide therapy should be observed for these signs or symptoms of fluid or electrolyte imbalance
(hyponatraemia, hypochloraemic alkalosis or hypokalaemia, hypomagnesaemia or hypocalcaemia) : dryness of mouth, thirst, weakness, lethargy, drowsiness, restlessness, muscle pains or cramps, muscular fatigue, hypotension, oliguria, tachycardia, arrhythmia, or gastrointestinal disturbances such as nausea and vomiting. Increases in blood glucose and alterations in glucose tolerance tests (with abnormalities of the fasting and 2-hour postprandial sugar) have been observed, and rarely, precipitation of diabetes mellitus has been reported. In patients with severe symptoms of urinary retention (because of bladder emptying disorders, prostatic hyperplasia, urethral narrowing), the administration of furosemide can cause acute urinary retention related to increased production and retention of urine. Thus, these patients require careful monitoring, especially during the initial stages of treatment. In patients at high risk for radiocontrast nephropathy, furosemide can lead to a higher incidence of deterioration in renal function after receiving radiocontrast compared to high-risk patients who received only intravenous hydration prior to receiving radiocontrast.
In patients with hypoproteinaemia (e.g., associated with nephrotic syndrome) the effect of furosemide may be weakened and its ototoxicity potentiated. Asymptomatic hyperuricaemia can occur and gout may rarely be precipitated. Patients allergic to sulfonamides may also be allergic to furosemide. The possibility exists of exacerbation or activation of systemic lupus erythematosus. As with many other drugs, patients should be observed regularly for the possible occurrence of blood dyscrasias, liver or kidney damage, or other idiosyncratic reactions. When FUROSEMIDE INJECTION USP is administered parenterally, a maximum injection or infusion rate of 4 mg/min (in normal renal function) or 2.5 mg/min (in impaired renal function) should be used to minimise the risk of ototoxicity.
Intramuscular administration of FUROSEMIDE INJECTION USP must be limited to exceptional cases where neither oral nor intravenous administrations are feasible. Intramuscular administration is not suitable for acute conditions such as pulmonary oedema. During long-term therapy, a high potassium diet is recommended (lean meat, potatoes, banana, tomatoes, cauliflower, spinach, dried fruit etc.). Potassium supplements may be required, especially when high doses are used for prolonged periods. Particular caution with potassium is necessary when the patient is on digitalis glycosides, potassium depleting steroids or in the case of infants and children. Potassium supplementation, diminution in dose, or discontinuation of furosemide therapy may be required.
Reversible elevations of blood urea may be seen. These have been observed in association with dehydration, which should be avoided, particularly in patients with renal insufficiency. Furosemide increases chlolesterol and triglycerides short-term. It is not clear whether this effect persists long term; however the current evidence does not indicate this. As with many other medicines, patients should be observed regularly for the possible occurrence of blood dyscrasias, liver damage, or other idiosyncratic reactions. Renal calcifications (from barely visible on X-ray to staghorn) have occurred in some severely premature infants treated with intravenous FUROSEMIDE INJECTION USP for oedema due to patent ductus arteriosus and hyaline membrane disease. The concurrent use of chlorothiazides has been reported to decrease hypercalciuria and to dissolve some calculi. The possibility exists of exacerbation or activation of systemic lupus erythematous.
Laboratory tests :
Serum electrolytes, (particularly potassium), CO2, creatinine and BUN should be determined frequently during the first few months of furosemide therapy and periodically thereafter. Serum and urine electrolyte determinations are particularly important when the patient is vomiting profusely or receiving parenteral fluids. Abnormalities should be corrected or the drug temporarily withdrawn. Other medications may also influence serum electrolytes. Reversible elevations of BUN may occur and are associated with dehydration, which should be avoided, particularly in patients with renal insufficiency. Urine and blood glucose should be checked periodically in diabetics receiving furosemide, even in those suspected of latent diabetes. Furosemide may lower serum levels of calcium (rarely cases of tetany have been reported) and magnesium. Accordingly, serum levels of these electrolytes should be determined periodically. In premature infants furosemide may precipitate nephrocalcinosis/ nephrolithiasis, therefore renal function must be monitored and renal ultrasonography performed.
Pregnancy : Category C
Furosemide crosses the placenta. Studies in humans have not been done. Studies in rabbits and mice have shown that furosemide causes an increased incidence of hydronephrosis in the foetus. In rabbits, unexplained maternal deaths and abortions have occurred at doses 2 to 8 times the maximum recommended human dose.
Nursing mothers :
Furosemide passes into breast milk and may inhibit lactation. Furosemide is not recommended in nursing mothers.
Paediatric use :
Caution is required in neonates because of the prolonged half-life of furosemide. Usual paediatric doses may be used, but the dosing interval should be extended.
EFFECTS ON ABILITY TO DRIVE AND USE MACHINES :
Some adverse effects (e.g. an undesirable pronounced fall in blood pressure) may impair the patient’s ability to concentrate and react and therefore constitute a risk in situations where these abilities are of special importance (e.g. operating a vehicle or machinery).
INTERACTIONS :
Alcohol :
Enhanced hypotensive effect. Orthostatic hypotension, associated with diuretics, may be enhanced.
Aldesleukin :
Enhanced hypotensive effect.
Anaesthetics, general :
Enhanced hypotensive effects.
Anion-exchange resins :
Colestyramine and colestipol markedly reduce the absorption of furosemide. Administer two to three hours apart.
Anti-arrhythmics :
Toxicity of amiodarone, disopyramide, flecainide and quinidine is increased if hypokalaemia occurs. Action of lidocaine and mexilitine is antagonised by hypokalaemia. Hypokalaemia increases risk of ventricular arrhythmias with sotalol, a beta-blocker.
Antibacterials :
Furosemide may enhance the toxicity of nephrotoxic antibiotics including some cephalosporins. It can enhance the ototoxicity of aminoglycoside antibiotics, vancomycin and other ototoxic agents. Since this may lead to permanent damage, these drugs must only be used with furosemide if there are compelling medical reasons.
Anticoagulants :
Reduced anticoagulant effect when furosemide used concomitantly with warfarin.
Antidepressants :
Increased risk of postural hypotension with tricyclic antidepressants. Enhanced hypotensive effect with monoamine oxidase inhibitors (MAOIs). Increase risk of hypokalaemia when furosemide and reboxetine are used concomitantly.
Antidiabetics :
The hypoglycaemic effect is antagonised by loop diuretics.
Antiepileptics :
Increased risk of hyponatraemia with concomitant carbamazepine. The diuretic effect of furosemide has been shown to be substantially reduced by concomitant phenytoin therapy.
Antifungals :
Increased risk of hypokalaemia with loop diuretics and amphotericin.
Anti-gout :
Probenecid has been shown to reduce the renal clearance of furosemide and may increase, decrease or have no effect on the overall diuresis. Furosemide may reduce the renal clearance of probenecid. In case of high-dose treatment (with furosemide and probenecid), this may lead to increased serum levels and an increased risk of adverse effects.
Antihistamines :
Hypokalaemia increases risk of ventricular arrhythmias with terfenadine.
Antihypertensives :
Furosemide enhances the hypotensive action of other antihypertensive drugs, including beta-blockers, calcium-channel blockers and hydralazine. The dosage of currently administered antihypertensive agents may require adjustment. There is an increase risk of first-dose hypotension with alpha blockers such as prazosin or angiotensin-converting enzyme (ACE) inhibitors such as captopril. Particular care should be taken with ACE inhibitors and angiotensin-II antagonists when initiating or increasing their dose in concomitant therapy with furosemide, since combination can result in marked reduction in blood pressure and deterioration in renal function. The dose of furosemide should be reduced for at least three days, or the drug stopped, before initiating or increasing the dose of an ACE inhibitor or angiotensin II receptor antagonist. Long term intensive treatment with captopril can enhance the natriuretic response to furosemide.
Antipsychotics :
Hypokalaemia increases risk of ventricular arrhythmias with primozide and sertindole, concurrent use should be avoided. Enhanced hypotensive effect with phenothiazines. Risperidone : Caution should be exercised and the risks and benefits of the combination or co-treatment with furosemide or with other potent diuretics should be considered prior to the decision to use.
Anxiolytics and hypnotics :
Administration of chloral hydrate followed by intravenous furosemide may result in a syndrome of hot flushes, sweating, tachycardia and hypertension.
Cardiac Glycosides :
Increased risk of toxicity if hypokalaemia or hypomagnesaemia occurs. The cardiac glycoside dosage may require adjustment as a more pronounced fall in blood pressure must be anticipated if given concomitantly with furosemide.
Corticosteroids :
The increased risk of hypokalaemia occurs particularly with the naturally occurring corticosteroids such as cortisone and hydrocortisone. The synthetic corticosteroids have a much less marked potassium-losing effect. Fluid retention associated with corticosteroid use may cause antagonism of diuretic/antihypertensive effect. Concomitant administration of corticosteroids may cause sodium retention.
Cytotoxics :
There is a risk of ototoxic effects if cisplatin and furosemide are given concomitantly. In addition, nephrotoxicity of cisplatin may be enhanced if furosemide is not given in low doses (e.g. 40 mg in patients with normal renal function) and with positive fluid balance when used to achieve forced diuresis during cisplatin treatment.
Diuretics :
Increased risk of hypokalaemia with other loop diuretics and other diuretics, including acetazolamide and thiazides. Severe electrolyte disturbances may occur in patients given metolazone concurrently with furosemide. The dosage of concurrently administered diuretics may require adjustment as a more pronounced fall in blood pressure must be anticipated if given concomitantly with furosemide.
Dopaminergics :
Enhanced hypotensive effect with levodopa.
Immunosuppressants :
Ciclosporin : concomitant use of ciclosporin and furosemide is associated with increased risk of gouty arthritis.
Laxatives :
Prolonged use may increase the risk of developing hypokalaemia.
Lithium :
In common with other diuretics, serum lithium levels may be increased when lithium is given concomitantly with furosemide, resulting in increased lithium toxicity, including increased risk of cardiotoxic and neurotoxic effects. It is recommended that lithium levels are carefully monitored and where necessary the lithium dosage is adjusted in patients receiving this combination.
Muscle relaxants :
Enhanced hypotensive effect may occur with tizanidine; effects of curare type muscle relaxants may be potentiated.
Nicotine :
Nicotine inhibits diuresis and diminishes the diuretic effect of furosemide.
Nitrates :
Enhanced hypotensive effect.
Non-steroidal anti-inflammatory agents (NSAIDs) :
Certain non-steroidal anti-inflammatory agents (e.g. indometacin, ketorolac, acetylsalicylic acid) may attenuate the diuretic effect of furosemide and may cause acute renal failure in cases of pre-existing hypovolaemia or dehydration. Enhanced salicylate toxicity or nephrotoxicity of NSAIDs.
Prostaglandins :
Hypotensive effect may be potentiated by alprostadil.
Sympathomimetics :
There is an increased risk of hypokalaemia with high doses of β2-sympathomimetics. Effects of pressor amines may be attenuated.
Theophylline :
Risk of hypokalaemia may be increased; effects of theophylline may be potentiated.
Ulcer healing drugs :
Carbenoxolone and liquorice may increase risk of hypokalaemia. Fluid retention associated with carbenoxolone may cause antagonism of diuretic/antihypertensive effect. Ranitidine causes a moderate increase in the bioavailability of furosemide.
INCOMPATIBILITIES :
Solutions of furosemide for injection are alkaline and should not be mixed or diluted with glucose injection or other acidic solutions. Furosemide injection has been reported to be visually incompatible with injections of diltiazem hydrochloride, dobutamine hydrochloride, dopamine hydrochloride, labetalol hydrochloride, midazolam hydrochloride, milrinone lactate, nicardipine hydrochloride, and vecuronium bromide. Incompatibility has also been noted with parenteral nutrient solutions, with cisatracurium besilate, with levofloxacin, with phenylephrine, and with vasopressin.
SIDE EFFECTS :
As with other diuretics, electrolytes and water balance may be disturbed during therapy with frusemide, especially in patients receiving high doses for a prolonged period. Excessive diuresis may give rise especially in elderly patients and children, to circulatory disturbances such as headache, dizziness, dry mouth or visual impairment, as symptoms of hypovolaemia. In extreme cases, hypovolaemia and dehydration may lead to hypotension, circulatory collapse and in elderly patients in particular, thrombophilia. However, with individualised dosage, acute haemodynamic reactions are generally not to be expected, although diuresis sets in rapidly. All saluretics may cause hypokalaemia, mainly in cases of low potassium diet, vomiting or chronic diarrhoea. Factors such as underlying diseases (liver cirrhosis, cardiac failure), concomitant medication or nutritional inadequacies (excessive restriction of salt intake), may lead to sodium or other electrolyte or fluid deficiencies which may produce a fall in orthostatic blood pressure, calf muscle spasms, anorexia, weakness, dizziness, drowsiness, apathy, vomiting and confusion. Furosemide may lower the serum calcium level. This may trigger a state of increased neuromuscular irritability. In very rare cases, tetany has been observed. In premature infants, calcium salts may be deposited in the renal tissue (nephrocalcinosis). Hypomagnesaemia and in rare cases, tetany or cardiac arrhythmia have been observed as a consequence of increased renal magnesium losses. Treatment with furosemide may lead to transitory increases in blood creatinine and urea levels and to an increase in cholesterol and triglyceride serum levels. Serum levels of uric acid may increase and attacks of gout may occur.
Hepatic System :
Isolated cases of acute pancreatitis and increases in liver transaminases have been observed. Additionally, intrahepatic cholestasis and jaundice have been reported, however, relationship to the medicine has not been established. Furosemide may increase the bile flow and distend the biliary tree, which is already obstructed.
Central Nervous System :
Reactions such as dizziness, vertigo, paraesthesia, headache and blurred vision occasionally accompany FUROSEMIDE INJECTION USP induced diuresis. Tinnitus, reversible impairment and rarely, permanent impairment of hearing have been observed with markedly reduced renal function or hypoproteinaemia. (e.g. in nephrotic syndrome). This occurs particularly when the recommended rate of injection or infusion of 4 mg/min (normal renal function) or 2.5 mg/min (impaired renal function) is exceeded, or in patients who are also
receiving medicines known to be ototoxic.
Dermatologic :
Allergic reactions may occasionally occur in the form of dermatitis, including rash, urticaria and rare cases of exfoliative dermatitis, necrotising angitis, bullous eruption, erythema multiforme and purpura and pruritus. Photosensitivity reactions have occasionally been reported.
Haematologic :
The following rare adverse reactions have been reported : eosinophilia, thrombophlebitis, haemolytic or aplastic anaemia, leucopenia, thrombocytopenia, agranulocytosis. Vasculitis may also occur.
Urinary System :
Excessive diuresis and dehydration could cause transient elevation of creatinine and BUN and reduction of GFR. In elderly men with prostatic hypertrophy, acute urinary retention with overflow incontinence may occur. Interstitial nephritis has also been reported with furosemide use. Symptoms of existing obstructed micturition in patients with conditions such as uretostenosis or hydronephrosis may be triggered or aggravated by pronounced diuresis.
Cardiovascular :
Orthostatic hypotension may occur and may be aggravated by alcohol, narcotics and barbiturates. Ischaemic complications have also been reported in elderly patients.
Other :
Restlessness, fever, rise in serum cholesterol and triglyceride, transient pain at the injection site following intramuscular injection. Treatment with furosemide has occasionally caused reduced glucose tolerance and deterioration in cases of manifest diabetes, or made latent diabetes manifest. Pre-existing metabolic alkalosis (e.g. due to decompensated liver cirrhosis) may be aggravated during furosemide treatment. Uricaemia may occur and lead to gout attacks in predisposed patients. Rarely, fever or paraesthesiae and occasionally photosensitivity may occur. In premature infants, Furosemide may precipitate nephrocalcinosis/ nephrolithiasis. If furosemide is administered to premature infants during the first weeks of life, it may increase the risk of persistence of patent ductus arteriosus. Following intramuscular injection, local reactions such as pain may occur. Due to the possibility of side effects such as hypotension, patients ability to drive or operate machinery may be impaired, especially at the commencement of therapy. Anaphylactic shock is rare, but is acutely life-threatening if it does occur. Whenever adverse reactions are moderate or severe, Furosemide dose should be reduced or therapy withdrawn.
INFORMATION FOR PATIENTS :
Patients receiving furosemide should be advised that they may experience symptoms from excessive fluid and/or electrolyte losses. The postural hypotension that sometimes occurs can usually be managed by getting up slowly. Potassium supplements and/or dietary measures may be needed to control or avoid hypokalaemia. Patients with diabetes mellitus should be told that furosemide may increase blood glucose levels and thereby affect urine glucose tests. The skin of some patients may be more sensitive to the effects of sunlight while taking furosemide. Hypertensive patients should avoid medications that may increase blood pressure, including over-the-counter products for appetite suppression and cold symptoms.
OVERDOSAGE :
The clinical picture in acute or chronic overdose depend primarily on the extent and consequences of electrolyte and fluid loss; e.g. dehydration, blood volume reduction, hypotension, electrolyte imbalance, cardiac arrhythmias (including A-V block and ventricular fibrillation), hypokalaemia and hypochloraemic alkalosis, and extensions of its diuretic action. Symptoms of these disturbances include severe hypotension (progressing to shock), acute renal failure, thrombosis, delirious states, flaccid paralysis, apathy and confusion. The acute toxicity of Frusemide has been determined in mice, rats and dogs. The intravenous LD50 ranged from 300 to 680 mg/kg. The concentration of furosemide in biological fluids associated with toxicity or death is not known.
TREATMENT OF OVERDOSAGE :
No specific antidote to furosemide is known. Treatment of overdosage is supportive. Serum electrolytes, carbon dioxide level and blood pressure should be determined frequently. Adequate drainage must be assured in patients with urinary bladder outlet obstruction (such as prostatic hypertrophy). Haemodialysis does not accelerate furosemide elimination.
PHARMACEUTICAL PRECAUTIONS :
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
STORAGE :
Store below 30°C (86°F), protected from light. Do not refrigerate.
SHELF LIFE :
24 months from the date of manufacture.
PRESENTATION :
FUROSEMIDE INJECTION USP is supplied as 20 mg of Furosemide USP in 2 ml aqueous solution.
5 Ampoules per Box.
Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.

 Cardiovascular
Cardiovascular





