250 mg
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
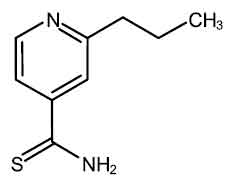
PROTHIONAMIDE TABLETS I.P. (Prothionamide) is used in the treatment of tuberculosis. It is a second-line anti-tubercular agent. Chemically, Prothionamide is 2-Propyl-4-pyridinecarbothioamide. The molecular formula is C9H12N2S and the molecular weight is 180.3.
STRUCTURAL FORMULA :
Its structural formula is :
PROTHIONAMIDE TABLETS I.P. is a yellow coloured, round film coated tablet.
COMPOSITION :
Each film coated tablet contains :
Prothionamide I.P. 250 mg
Excipients q.s.
Colour : Titanium Dioxide I.P., Tartrazine, Sunset Yellow.
ACTIONS :
Antibacterial Activity :
Prothionamide is a bacteriostatic drug which acts by inhibiting protein synthesis in the cell and inhibits mycolic acid synthesis. The multiplication of Mycobacterium tuberculosis is suppressed by concentrations of prothionamide ranging from 0.6 to 2.5 mg/ml. Resistance can develop rapidly in vitro and in vivo. A concentration of 10 mg/ml or less will inhibit approximately 75 % of photochromogenic mycobacteria; the scotochromogens are more resistant. M. tuberculosis strains which have become resistant to drugs such as isoniazid, streptomycin and PAS remain Prothionamide -sensitive.
MICROBIOLOGY :
In Vitro Activity :
Prothionamide exhibits bacteriostatic activity against extracellular and intracellular Mycobacterium tuberculosis organisms. The development of prothionamide resistant M. tuberculosis isolates can be obtained by repeated subculturing in liquid or on solid media containing increasing concentrations of prothionamide. Multi-drug resistant strains of M. tuberculosis may have acquired resistance to both isoniazid and prothionamide. However, the majority of M. tuberculosis isolates that are resistant to one are usually susceptible to the other. There is no evidence of cross-resistance between prothionamide and para-aminosalicylic acid (PAS), streptomycin, or cycloserine. However, limited data suggest that cross-resistance may exist between prothionamide and thiosemicarbazones (i.e., thiacetazone) as well as isoniazid.
In Vivo Activity :
Prothionamide administered orally initially decreased the number of culturable Mycobacterium tuberculosis organisms from the lungs of H37Rv infected mice. Drug resistance developed with continued prothionamide monotherapy, but did not occur when mice received prothionamide in combination with streptomycin or isoniazid.
SUSCEPTIBILITY TESTING :
Prothionamide susceptibility testing should only be performed by qualified or reference laboratories. Two standardized in vitro susceptibility methods are available for testing prothionamide against M. tuberculosis organisms. The modified proportion method (CDC or NCCLS M24-P) utilizes Middlebrook and Cohn 7H10 agar medium impregnated with prothionamide at a final concentration of 5.0 µg/mL. After 2 to 3 weeks of incubation, MIC99 values are calculated by comparing the quantity of organisms growing in the medium containing drug to the control cultures. Mycobacterial growth in the presence of drug, of at least 1 % of the growth in the control culture, indicates resistance. The radiometric broth method employs the BACTEC 460 machine to compare the growth index from untreated control cultures to cultures grown in the presence of 5.0 µg/mL of prothionamide. Strict adherence to the manufacturer’s instructions for sample processing and data interpretation is required for this assay. Susceptibility test results obtained by these two different methods cannot be compared unless equivalent drug concentrations are evaluated. The clinical relevance of in vitro susceptibility test results for mycobacterial species other than M. tuberculosis using either the radiometric or the proportion method has not been determined.
PHARMACOKINETICS :
Prothionamide is readily absorbed from the gastrointestinal tract and produces peak plasma concentrations about 2 hours after an oral dose. It is widely distributed throughout body tissues and fluids, including the CSF. Prothionamide is metabolised to the active sulfoxide and other inactive metabolites and less than 1 % of a dose appears in the urine as unchanged drug.
INDICATIONS :
PROTHIONAMIDE TABLETS I.P. is primarily indicated for the treatment of active tuberculosis in patients with M. tuberculosis resistant to isoniazid or rifampin, or when there is intolerance on the part of the patient to other drugs. Its use alone in the treatment of tuberculosis results in the rapid development of resistance. It is essential, therefore, to give a suitable companion drug or drugs, the choice being based on the results of susceptibility tests. If the susceptibility tests indicate that the patient’s organism is resistant to one of the first-line antituberculosis drugs (i.e., isoniazid or rifampin) yet susceptible to prothionamide, prothionamide should be accompanied by at least one drug to which the M. tuberculosis isolate is known to be susceptible. If the tuberculosis is resistant to both isoniazid and rifampin, yet susceptible to prothionamide, prothionamide should be accompanied by at least two other drugs to which the M. tuberculosis isolate is known to be susceptible.
Patient nonadherence to prescribed treatment can result in treatment failure and in the development of drug-resistant tuberculosis, which can be life-threatening and lead to other serious health risks. It is, therefore, essential that patients adhere to the drug regimen for the full duration of treatment. Directly observed therapy is recommended for all patients receiving treatment for tuberculosis. Patients in whom drug-resistant M. tuberculosis organisms are isolated should be managed in consultation with an expert in the treatment of drug-resistant tuberculosis.
Administration :
PROTHIONAMIDE TABLETS I.P. is administered orally.
Dosage :
In the treatment of tuberculosis, a major cause of the emergence of drug-resistant organisms and thus treatment failure, is patient nonadherence to prescribed treatment. Treatment failure and drug-resistant organisms can be life-threatening and may result in other serious health risks. It is, therefore, important that patients adhere to the drug regimen for the full duration of treatment. Directly observed therapy is recommended when patients are receiving treatment for tuberculosis. Consultation with an expert in the treatment of drug-resistant tuberculosis is advised for patients in whom drug-resistant tuberculosis is suspected or likely. Prothionamide should be administered with at least one, sometimes two, other drugs to which the organism is known to be susceptible.
Therapy should be initiated at a dose of 250 mg daily, with gradual titration to optimal doses as tolerated by the patient. A regimen of 250 mg daily for 1 or 2 days, followed by 250 mg twice daily for 1 or 2 days with a subsequent increase to 1 gm in 3 or 4 divided doses has been reported. Thus far, there is insufficient evidence to indicate the lowest effective dosage levels. Therefore, in order to minimize the risk of resistance developing to the drug or to the companion drug, the principle of giving the highest tolerated dose (based on gastrointestinal intolerance) has been followed. In the adult this would seem to be between 0.5 and 1.0 gm daily, with an average of 0.75 gm daily.
The optimum dosage for paediatric patients has not been established. However, paediatric dosages of 10 to 20 mg/kg p.o. daily in 2 or 3 divided doses given after meals or 15 mg/kg/24 hrs as a single daily dose have been recommended. As with adults, Prothionamide may be administered to paediatric patients once daily. It should be noted that in patients with concomitant tuberculosis and HIV infection, malabsorption syndrome may be present. Drug malabsorption should be suspected in patients who adhere to therapy, but who fail to respond appropriately. In such cases, consideration should be given to therapeutic drug monitoring.
The best times of administration are those which the individual patient finds most suitable in order to avoid or minimize gastrointestinal intolerance, which is usually at mealtimes. Every effort should be made to encourage patients to persevere with treatment when gastrointestinal side effects appear, since they may diminish in severity as treatment proceeds. Concomitant administration of pyridoxine is recommended. Duration of treatment should be based on individual clinical response. In general, continue therapy until bacteriological conversion has become permanent and maximal clinical improvement has occurred.
CONTRAINDICATIONS :
Severe hypersensitivity to Prothionamide.
Severe hepatic damage.
PROTHIONAMIDE TABLETS I.P. contains lactose which is contra-indicated in patients with galactosaemia, the glucose-galactose malabsorption syndrome or lactase deficiency.
WARNINGS :
The use of PROTHIONAMIDE TABLETS I.P. alone in the treatment of tuberculosis results in rapid development of resistance. It is essential, therefore, to give a suitable companion drug or drugs, the choice being based on the results of susceptibility testing. However, therapy may be initiated prior to receiving the results of susceptibility tests as deemed appropriate by the physician. Prothionamide should be administered with at least one, sometimes two, other drugs to which the organism is known to be susceptible. Drugs which have been used as companion agents are rifampin, ethambutol, pyrazinamide, cycloserine, kanamycin, streptomycin and isoniazid. The usual warnings, precautions, and dosage regimens for these companion drugs should be observed.
Patient compliance is essential to the success of the antituberculosis therapy and to prevent the emergence of drug-resistant organisms. Therefore, patients should adhere to the drug regimen for the full duration of treatment. It is recommended that directly observed therapy be practiced when patients are receiving antituberculous medication. Additional consultation from experts in the treatment of drug-resistant tuberculosis is recommended when patients develop drug-resistant organisms. The tablet contains “tartrazine” colour which may cause allergic reactions, including bronchial asthma.
PRECAUTIONS :
General :
Prothionamide may potentiate the adverse effects of the other antituberculous drugs administered concomitantly. Ophthalmologic examinations (including ophthalmoscopy) should be performed before and periodically during therapy with PROTHIONAMIDE TABLETS I.P. PROTHIONAMIDE TABLETS I.P. should be used cautiously in diabetic patients.
Laboratory Tests :
Determination of serum transaminases (SGOT, SGPT) should be made prior to initiation of therapy and should be monitored monthly. If serum transaminases become elevated during therapy, Prothionamide and the companion antituberculosis drug or drugs may be discontinued temporarily until the laboratory abnormalities have resolved. Prothionamide and the companion antituberculosis medication(s) then should be reintroduced sequentially to determine which drug (or drugs) is (are) responsible for the hepatotoxicity. Blood glucose determinations should be made prior to and periodically throughout therapy with PROTHIONAMIDE TABLETS I.P. Diabetic patients should be particularly alert for episodes of hypoglycaemia. Periodic monitoring of thyroid function tests is recommended as hypothyroidism, with or without goiter, has been reported with Prothionamide therapy.
Use in pregnancy : Category C
Teratogenic effects have been demonstrated in small animals receiving doses in excess of those recommended for human beings. Use of the drug should be avoided during pregnancy or in women of childbearing potential unless the benefits outweigh its possible hazard.
Nursing mothers :
Because no information is available on the excretion of prothionamide in human milk, PROTHIONAMIDE TABLETS I.P. should be administered to nursing mothers only if the benefits outweigh the risks. Newborns who are breast-fed by mothers who are taking PROTHIONAMIDE TABLETS I.P. should be monitored for adverse effects.
Paediatric use :
Due to the fact that pulmonary tuberculosis resistant to primary therapy is rarely found in neonates, infants and children, investigations have been limited in these age groups. At present, the drug should not be used in paediatric patients under 12 years of age except when the organisms are definitely resistant to primary therapy and systemic dissemination of the disease or other life-threatening complications of tuberculosis is judged to be imminent.
INTERACTIONS :
Prothionamide may intensify the adverse effects of other antitubercular drugs administered concomitantly. Convulsions have been reported and special care should be taken particularly when prothionamide is administered with cycloserine. Excessive ethanol ingestion should be avoided because a psychotic reactions has been reported.
SIDE EFFECTS :
Gastrointestinal : The most common side effects of prothionamide are gastrointestinal disturbances including nausea, vomiting, diarrhoea, abdominal pain, excessive salivation, metallic taste, stomatitis, anorexia and weight loss. Adverse gastrointestinal effects appear to be dose related, with approximately 50 % of patients unable to tolerate 1 gm as a single dose. Gastrointestinal effects may be minimized by decreasing dosage, by changing the time of drug administration or by the concurrent administration of an antiemetic agent.
Nervous System : Psychotic disturbances (including mental depression), drowsiness, dizziness, restlessness, headache and postural hypotension have been reported with prothionamide. Rare reports of peripheral neuritis, optic neuritis, diplopia, blurred vision, and a pellagra-like syndrome also have been reported. Concurrent administration of pyridoxine has been recommended to prevent or relieve neurotoxic effects.
Hepatic : Transient increases in serum bilirubin, SGOT, SGPT; Hepatitis (with or without jaundice).
Other : Hypersensitivity reactions including rash, photosensitivity, thrombocytopenia and purpura have been reported rarely. Hypoglycaemia, gynaecomastia, impotence, and acne also have occurred. The management of patients with diabetes mellitus may become more difficult in those receiving prothionamide.
INFORMATION FOR PATIENTS :
Patients should be advised to consult their physician should blurred vision or any loss of vision, with or without eye pain, occur during treatment. Excessive ethanol ingestion should be avoided because a psychotic reaction has been reported.
OVERDOSAGE AND TREATMENT OF OVERDOSAGE :
Acute overdosage may lead to an accentuation of the symptoms listed under SIDE EFFECTS. Treatment is symptomatic and supportive.
STORAGE :
Store below 25°C (77°F), protected from moisture and light.
Do not refrigerate.
SHELF LIFE :
24 months from the date of manufacture.
PRESENTATION :
PROTHIONAMIDE TABLETS I.P. contain Prothionamide I.P. 250 mg.
10 Blisters of 10 Tablets Per box.
Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.
 Cardiovascular
Cardiovascular