20 mg/ml
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
HYDRALAZINE HYDROCHLORIDE INJECTION USP (Hydralazine hydrochloride) is a peripheral vasodilator. Chemically, Hydralazine hydrochloride is 1-Hydrazinophthalazine monohydrochloride. The molecular formula is C8H8N4·HCl and molecular weight is 196.64
STRUCTURAL FORMULA :
Its structural formula is :
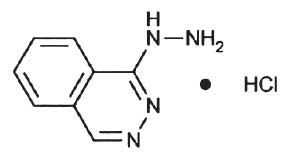
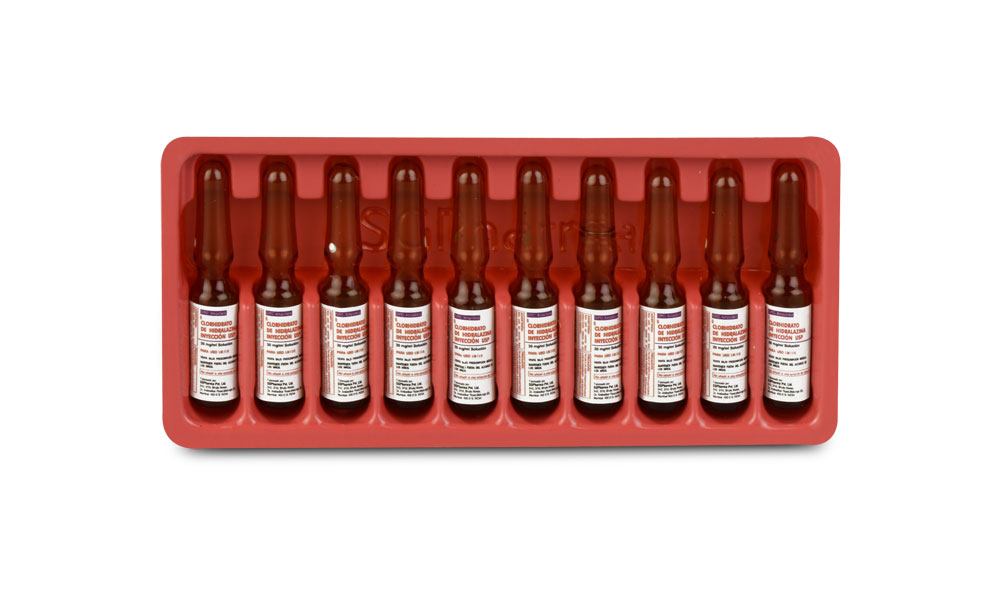
HYDRALAZINE HYDROCHLORIDE INJECTION USP is a sterile, clear, nonpyrogenic, colourless solution filled in 1 ml Amber Ampoule.
Administration :
HYDRALAZINE HYDROCHLORIDE INJECTION USP is for I.M./I.V. administration.
INSTRUCTIONS FOR USE OF AMPOULE :
The ampoule used in this product is equipped with O.P.C (One Point Cut) opening system. No ampoule file is needed to open the ampoule. The neck of the ampoule is prescored at the point of constriction. A coloured dot on the ampoule head helps to orientate the ampoule. Take the ampoule and face the coloured dot. Let the solution at the head of the ampoule to flow down by shaking or a gentle stroke. The ampoule opens easily by placing the thumb on the coloured dot and gently pressing downwards as shown.

Discoloration of HYDRALAZINE HYDROCHLORIDE INJECTION USP was observed on several occasions after storage in syringe for upto twelve hours. Hydralazine reacts with metals and therefore Injection should be prepared with a nonmetallic filter and should be used as quickly as possible after being drawn through needle in syringe.
Dosage :
When there is urgent need, therapy in the hospitalized patient may be initiated intramuscularly or as a rapid intravenous bolus injection directly into the vein. Hydralazine Hydrochloride Injection should be used only when the drug cannot be given orally. The usual dose is 20 to 40 mg, repeated as necessary. Certain patients (especially those with marked renal damage) may require a lower dose. Blood pressure should be checked frequently. It may begin to fall within a few minutes after injection, with the average maximal decrease occurring in 10 to 80 minutes. In cases where there has been increased intracranial pressure, lowering the blood pressure may increase cerebral ischemia. Most patients can be transferred to oral hydralazine hydrochloride within 24 to 48 hours. The product should be used immediately after the ampoule is opened. The product should not be added to infusion solutions. HYDRALAZINE HYDROCHLORIDE INJECTION USP may discolor upon contact with metal; discolored solutions should be discarded.
CONTRAINDICATIONS :
Known hypersensitivity to hydralazine or dihydralazine.
Idiopathic systemic lupus erythematosus (SLE) and related diseases.
Severe tachycardia and heart failure with a high cardiac output (e.g. in thyrotoxicosis).
Myocardial insufficiency due to mechanical obstruction (e.g. in the presence of aortic or mitral stenosis or constrictive pericarditis).
Isolated right ventricular failure due to pulmonary hypertension.
WARNINGS :
The overall ‘hyperdynamic’ state of the circulation induced by hydralazine may accentuate certain clinical conditions. Myocardial stimulation may provoke or aggravate angina pectoris. Patients with suspected or confirmed coronary artery disease should therefore be given Hydralazine Injection only under cover of beta-blocker or in combination with other suitable sympatholytic agents. It is important that the beta-blocker medication should be commenced a few days before the start of treatment with HYDRALAZINE HYDROCHLORIDE INJECTION USP. Patients who have survived a myocardialinfarction should not receive HYDRALAZINE HYDROCHLORIDE INJECTION USP until a post-infarction stabilisation state has been achieved. Prolonged treatment with hydralazine (i.e. usually for more than 6 months) may provoke a lupus erythematosus (LE) like syndrome, especially where doses exceed 100 mg daily. First symptoms are likely to be arthralgia, sometimes associated with fever and rash and are reversible after withdrawal of the drug. In its more severe form it resembles acute SLE, and in rare cases renal and ocular involvements have been reported. Long term treatment with corticosteroids may be required to reverse these changes. Since such reactions tend to occur more frequently the higher the dose and the longer its duration, and since they are also more common in slow acetylators, it is recommended that for maintenance therapy the lowest effective dose should be used. If 100 mg daily fails to elicit an adequate clinical effect, the patient’s acetylator status should be evaluated. Slow acetylators and women run greater risk of developing the LE like syndrome and every effort should therefore be made to keep the dosage below 100 mg daily and a careful watch kept for signs and symptoms suggestive of this syndrome. If such symptoms do develop the drug should be gradually withdrawn. Rapid acetylators often respond inadequately even to doses of 100 mg daily and therefore the dose can be raised with only a slightly increased risk of an LE like syndrome.
During long term treatment with HYDRALAZINE HYDROCHLORIDE INJECTION USP it is advisable to determine the antinuclear factors and conduct urine analysis at intervals of approximately 6 months. Microhaematuria and / or proteinuria, in particular together with positive titres of ANF, may be initial signs of immune-complex glomerulonephritis associated with the SLE like syndrome. If overt clinical signs or symptoms develop, the drug should be withdrawn immediately. Skin rash, febrile reactions and change in blood count occur rarely and drug should be withdrawn. Peripheral neuritis in the form of paraesthesia has been reported, and may respond to pyridoxine administration or drug withdrawal.
PRECAUTIONS :
In patients with renal impairment (creatinine clearance < 30 ml/min or serum creatinine concentrations > 2.5 mg / 100 ml or 221 μmol/l) and in patients with hepatic dysfunction the dose or interval between doses should be adjusted according to clinical response, in order to avoid accumulation of the ‘apparent’ active substance. HYDRALAZINE HYDROCHLORIDE INJECTION USP should be used with caution in patients with coronary artery disease (since it may increase angina) or cerebrovascular disease. When undergoing surgery, patients treated with HYDRALAZINE HYDROCHLORIDE INJECTION USP may show a fall in blood pressure, in which case one should not use adrenaline to correct the hypotension, since it enhances the cardiac-accelerating effects of hydralazine. When initiating therapy in heart failure, particular caution should be exercised and the patient kept under surveillance and/or haemodynamic monitoring for early detection of postural hypotension or tachycardia. Where discontinuation of therapy in heart failure is indicated, HYDRALAZINE HYDROCHLORIDE INJECTION USP should be withdrawn gradually (except in serious situations, such as SLE-like syndrome or blood dyscrasias) in order to avoid precipitation and/or exacerbation of heart failure.
Pregnancy : Pregnancy category C
Animal studies indicate that hydralazine is teratogenic in mice at 20 to 30 times the maximum daily human dose of 200 to 300 mg and possibly in rabbits at 10 to 15 times the maximum daily human dose, but that it is nonteratogenic in rats. Teratogenic effects observed were cleft palate and malformations of facial and cranial bones. There are no adequate and well-controlled studies in pregnant women. Although clinical experience does not include any positive evidence of adverse effects on the human foetus, hydralazine should be used during pregnancy only if the expected benefit justifies the potential risk to the foetus.
Nursing mothers :
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when hydralazine injection is administered to a nursing woman.
Paediatric use :
Safety and effectiveness in paediatric patients have not been established in controlled clinical trials, although there is experience with the use of hydralazine hydrochloride in paediatric patients. The usual recommended parenteral dosage, administered intramuscularly or intravenously, is 1.7 to 3.5 mg/kg of body weight daily, divided into four to six doses.
INTERACTIONS AND INCOMPATIBILITIES :
Potentiation of effects : Concurrent therapy with other antihypertensives (vasodilators, calcium antagonists, ACE inhibitors, diuretics), anaesthetics, tricyclic antidepressants, major tranquillisers or drugs exerting central depressant actions (including alcohol). Administration of HYDRALAZINE HYDROCHLORIDE INJECTION USP shortly before or after diazoxide may give rise to marked hypotension. MAO inhibitors should be used with caution in patients receiving Hydralazine Injection. Concurrent administration of HYDRALAZINE HYDROCHLORIDE INJECTION USP with beta-blockers subject to a strong first pass effect (e.g. propranolol) may increase their bioavailability. Downward adjustment of these drugs may be required when they are given concomitantly with HYDRALAZINE HYDROCHLORIDE INJECTION USP.
SIDE EFFECTS :
Adverse reactions with HYDRALAZINE HYDROCHLORIDE INJECTION USP are usually reversible when dosage is reduced. However, in some cases it may be necessary to discontinue the drug. The following adverse reactions have been observed, but there has not been enough systematic collection of data to support an estimate of their frequency.
Common : Headache, anorexia, nausea, vomiting, diarrhoea, palpitations, tachycardia, angina pectoris.
Less Frequent : Digestive-constipation, paralytic ileus.
Cardiovascular - Hypotension, paradoxical pressor response, oedema.
Respiratory - Dyspnea.
Neurologic - Peripheral neuritis, evidenced by paresthesia, numbness, and tingling; dizziness; tremors; muscle cramps, psychotic reactions characterized by depression, disorientation, or anxiety.
Genitourinary - Difficulty in urination.
Haematologic - Blood dyscrasias, consisting of reduction in haemoglobin and red cell count, leukopaenia, agranulocytosis, purpura; lymphadenopathy; splenomegaly.
Hypersensitive Reactions - Rash, urticaria, pruritus, fever, chills, arthralgia, eosinophilia, and rarely, hepatitis. Other-nasal congestion, flushing, lacrimation, conjunctivitis.
OVERDOSAGE :
The chief manifestations are cardiovascular disorders such as pronounced tachycardia and hypotension, which are accompanied by nausea, dizziness, and sweating, and which can result in circulatory collapse; also possible are myocardial ischaemia with angina pectoris and cardiac arrhythmias. Further signs and symptoms may include impairment of consciousness, headache, and vomiting, as well as possibly tremor, convulsions, oliguria, and hypothermia.
TREATMENT OF OVERDOSAGE :
There is no specific antidote.
Support of the cardiovascular system is of primary importance. Shock should be treated with plasma expanders. If possible, vasopressors should not be given, but if a vasopressor is required, care should be taken not to precipitate or aggravate cardiac arrhythmia. Tachycardia responds to beta blockers. Digitalization may be necessary, and renal function should be monitored and supported as required. No experience has been reported with extracorporeal or peritoneal dialysis.
PHARMACEUTICAL PRECAUTION AFTER OVERDOSAGE :
Parenteral drug products should be inspected visually for particulate matter and discolouration prior to administration, whenever solution and container permit.
STORAGE :
Store below 30°C (86°F), protected from light.
Do not refrigerate.
SHELF LIFE :
24 months from the date of manufacture.
PRESENTATION :
HYDRALAZINE HYDROCHLORIDE INJECTION USP is supplied as 20 mg of Hydralazine hydrochloride USP in 1 ml Solution.
10 Ampoules per Box.
Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.

 Cardiovascular
Cardiovascular