
300 IU/5 ml
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
CORTICOTROPHIN CARBOXYMETHYLCELLULOSE INJECTION BPC’73 [Corticotrophin (ACTH)] is a polypeptide hormone produced and secreted by the pituitary gland.Corticotrophin (ACTH or adrenocorticotrophic hormone) is a 39 amino acid peptide with chemical formula : H-Ser1-Tyr-Ser-Met-Glu-His-Phe-Arg-Trp-Gly10-Lys-Pro-Val-Gly-Lys-Lys-Arg-Arg-Pro-Val20-Lys-Val-Tyr-Pro-Asn-Gly-Ala-Glu-Asp-Glu30-Leu-Ala-Glu-Ala-Phe-Pro-Leu-Glu-Phe39-OH The molecular formula is C210H314N56O57S and molecular weight is 4567.15.
Its structural formula is :
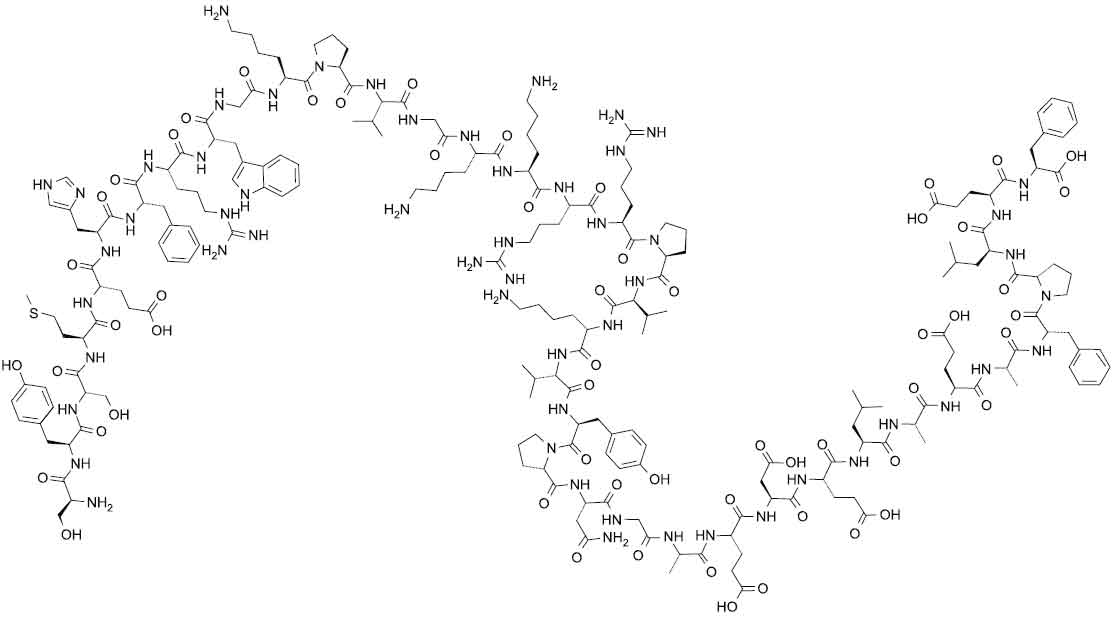
CORTICOTROPHIN CARBOXYMETHYLCELLULOSE INJECTION BPC’73 is a clear, colourless, slightly viscous liquid filled in a vial of suitable size.
COMPOSITION :
Each ml contains :
Corticotrophin (ACTH) 60 IU
Phenol B.P. 0.5 % w/v
(as preservative)
Water for injections B.P. q.s.
ACTIONS :
Diagnostic aid (adrenocortical function) - Corticotrophin combines with a specific receptor on the adrenal cell plasma membrane. In patients with normal adrenocortical function, it stimulates the initial reaction involved in the synthesis of adrenal steroids (including cortisol, cortisone, weak androgenic substances, and a limited quantity of aldosterone) from cholesterol by increasing the quantity of cholesterol within the mitochondria. Corticotrophin does not significantly increase serum cortisol concentrations in patients with primary adrenocortical insufficiency (Addison’s disease). Anticonvulsant (specific in infantile myoclonic seizures) - The mechanism of action of corticotrophin in the treatment of infantile myoclonic seizures is unknown.
Other actions/effects :
Corticotrophin is not a corticosteroid. However, it shares many actions of the corticosteroids due to its ability to increase endogenous corticosteroid synthesis.
PHARMACOKINETICS :
The ACTH in CORTICOTROPHIN CARBOXYMETHYLCELLULOSE INJECTION BPC’73 is of synthetic origin (porcine sequence). Reversible binding of ACTH to carboxymethylcellulose (CMC) protects the peptide against enzymatic breakdown and gives a prolonged clinical effect. Provided there is a normal adrenal cortex function, 2-3 injections per week of CORTICOTROPHIN CARBOXYMETHYLCELLULOSE INJECTION BPC’73 will give the expected clinical effect.
INDICATIONS :
- Diagnostic testing of adrenocortical function.
- Induce diuresis or remission of proteinuria in the nephrotic syndrome without uraemia of the idiopathic type or that caused by lupus erythematosus.
- Treatment of nonsuppurative thyroiditis.
- Hypercalcaemia associated with cancer.
- Acute exacerbations of multiple sclerosis.
- Tuberculous meningitis when accompanied by antituberculous chemotherapy.
- Trichinosis with neurologic or myocardial involvement.
- Treatment of glucocorticoid responsive rheumatic, collagenous, dermatologic, allergic, ophthalmic, respiratory, haematologic, neoplastic, and gastrointestinal diseases.
- Treatment of infantile spasms.
- Severe stress conditions.
- Ulcerative colitis.
- Chronic active liver disease.
- Hay fever.
Administration :
CORTICOTROPHIN CARBOXYMETHYLCELLULOSE INJECTION BPC’73 is given intramuscularly or subcutaneously; it must not be given intravenously.
Dosage :
The dosage is always individual depending on the nature of the disease and function of the adrenal cortex.
Long term treatment :
Adults :
For most patients 15-30 IU (0.25-0.5 ml) subcutaneously every two days is an effective dose for protracted treatment. If there is no effect it may be necessary to increase the dose of CORTICOTROPHIN CARBOXYMETHYLCELLULOSE INJECTION BPC’73 to 60 IU (1 ml) daily or every two days. The same effect as with 15 IU daily is also achieved with 30 IU every two days or 60 IU twice weekly. In certain diseases like severe dermatological disorders it may be necessary to administer 60 IU or more daily.
Infants :
In infantile spasm, 20 units IM daily for 2 weeks, if no response occurs, the dose is increased to 30 and then 40 units IM dose additional 4 weeks.
Short term treatment :
The dosage in short term or initial treatment may vary considerably, daily dose of up to 120 IU (2 ml) subcutaneously for intensive treatment, for example in multiple sclerosis, myasthenia gravis and uncreative colitis.
Note :
Body weight, blood pressure and appearance of eventual signs of a cushinoid state should be checked regularly during treatment of CORTICOTROPHIN CARBOXYMETHYLCELLULOSE INJECTION BPC’73.
CONTRAINDICATIONS :
CORTICOTROPHIN CARBOXYMETHYLCELLULOSE INJECTION BPC’73 is contraindicated in patients with :
- Scleroderma
- Osteoporosis
- Systemic fungal infections
- Ocular herpes simplex
- Recent surgery
- History or presence of peptic ulcer
- Congestive heart failure
- Hypertension
- Sensitivity to porcine proteins
- Conditions accompanied by primary adrenocortical insufficiency or adrenocortical hyperfunction.
- Intravenous administration is contraindicated.
Chronic administration of corticotrophin may lead to adverse effects which are not reversible. Corticotrophin may only suppress symptoms and signs of chronic diseases without altering the natural course of the disease. CORTICOTROPHIN CARBOXYMETHYLCELLULOSE INJECTION BPC’73 should not be administered for treatment until adrenal responsiveness has been verified with the route of administration which will be utilized during treatment, intramuscularly or subcutaneously. A rise in urinary and plasma corticosteroid values provides direct evidence of a stimulatory effect. Prolonged administration of corticotrophin increases the risk of hypersensitivity reactions. Although the action of corticotrophin is similar to that of exogenous adrenocortical steroids, the quantity of adrenocorticoid may be variable. In patients who receive prolonged corticotrophin therapy, the additional use of rapidly acting corticosteroids before, during, and after an unusual stressful situation is indicated. Prolonged use of corticotrophin may produce posterior subcapsular cataracts and glaucoma with possible damage to the optic nerves.
Corticotrophin may mask some signs of infection, and new infections including those of the eye due to fungi or viruses may appear during its use. There may be decreased resistance and inability to localize infection when corticotrophin is used. Corticotrophin can cause elevation of blood pressure, salt and water retention, and increased excretion of potassium. Dietary salt restriction and potassium supplementation may be necessary. Corticotrophin increases calcium excretion. While on corticotrophin therapy, patients should not be vaccinated against smallpox. Other immunization procedures should be undertaken with caution in patients who are receiving corticotrophin, especially when high doses are administered because of the possible hazards of neurological complications and lack of antibody response.
PRECAUTIONS :
General :
CORTICOTROPHIN CARBOXYMETHYLCELLULOSE INJECTION BPC’73 should be used in the lowest dose for the shortest period of time to accomplish the therapeutic goal. Corticotrophin should be used for treatment only when the disease is intractable to non-steroid treatment. There is an enhanced effect in patients with hypothyroidism and in those with cirrhosis of the liver. Sensitivity to porcine protein should be considered before starting therapy and during the course of treatment should symptoms arise. When an infection is present, appropriate antibiotic therapy should be given. Patients with latent tuberculosis should be observed closely, and if therapy is prolonged, chemoprophylaxis should be instituted. Psychic symptoms may appear with use of corticotrophin or pre-existing symptoms may be enhanced. These may range from mood alteration to a psychotic state. Patients with a secondary disease may have that disease worsened. Caution should be used when prescribing corticotrophin in patients with diabetes, renal insufficiency, diverticulitis, and myasthenia gravis.
Corticotrophin often acts by suppressing symptoms without altering the course of the underlying disease. Since complications with corticotrophin use are dependent on the dose and duration of treatment, a risk/benefit decision must be made in each case. Suppression of the pituitary adrenal axis occurs following prolonged therapy which may be slow in returning to normal. Patients should be protected from the stress of trauma or surgery by the use of corticosteroids during the period of stress. Since maximal corticotrophin stimulation of the adrenals may be limited during the first few days of treatment, other drugs should be administered when an immediate therapeutic effect is desirable. Although controlled clinical trials have shown ACTH to be effective in speeding the resolution of acute exacerbations of multiple sclerosis, they do not show that it affects the ultimate outcome or natural history of the disease. The studies do show that relatively high doses of ACTH are necessary to demonstrate a significant effect. Treatment of acute gouty arthritis should be limited to a few days. Since rebound attacks may occur when corticotrophin is discontinued, conventional concomitant therapy should be administered during corticotrophin treatment and for several days after it is stopped. Aspirin should be used cautiously in conjunction with corticotrophin in hypoprothrombinaemia.
Carcinogenesis, Mutagenesis, Impairment of Fertility :
Adequate and well-controlled studies have not been done in animals. Human use has not been associated with an increase in malignant disease.
Pregnancy : Category C
Studies in animals have shown that corticotrophin is embryocidal. Adequate and well-controlled studies have not been done in humans, but potential benefits may warrant use of the drug in pregnant women despite potential risks.
Nursing mothers :
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from corticotrophin, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Paediatric Use :
Prolonged use of corticotrophin in children will inhibit skeletal growth. If use is necessary, it should be given intermittently and the child carefully observed.
INTERACTIONS AND INCOMPATIBILITIES :
Anticholinesterases :
Effects of these agents may be antagonized in myasthenia gravis. Monitor the clinical response and provide mechanical respiratory support if needed.
Anticoagulants :
The hypoprothrombinaemic effect of anticoagulants may be altered unpredictably. Close clinical monitoring for signs of excessive hypoprothrombinaemia or anticoagulant failure is warranted. If an interaction is suspected, adjust the anticoagulant dose as needed.
Diuretic therapy (e.g., thiazides [e.g., hydrochlorothiazide]) :
Electrolyte loss associated with diuretic therapy may be accentuated. Monitor electrolytes and supplement if needed.
Interleukin-2 :
The pharmacologic effects of interleukin-2 may be decreased. Avoid co-administration.
Itraconazole :
Corticotrophin plasma concentrations and pharmacologic effects may be increased. The adrenal-suppressant effect of corticotrophin may be enhanced. Close clinical monitoring is warranted.
Mifepristone :
Co-administration is contraindicated.
Ritodrine :
The risk of maternal pulmonary oedema may be increased. If maternal pulmonary oedema develops, ritodrine should be stopped.
Vaccines :
Co-administration of live or live attenuated vaccines is contraindicated. Killed or inactivated vaccines may be given; however, the response to such vaccines cannot be predicted because of possible neurologic complications or lack of response.
SIDE EFFECTS :
Cardiovascular :
Hypertension, chronic heart failure, necrotizing angiitis.
Central Nervous System :
Convulsions, vertigo, headache, increased intracranial pressure with papilloedema, pseudotumor cerebri.
Dermatologic :
Impaired wound healing, petechiae and ecchymoses, increased sweating, hyperpigmentation, thin, fragile skin, facial erythema, acne.
Eye, Ear, Nose and Throat :
Posterior subcapsular cataracts, increased IOP (Intraocular pressure), glaucoma with possible optic nerve damage, exophthalmos.
Gastrointestinal :
Pancreatitis, ulcerative oesophagitis, abdominal distention, peptic ulcer.
Metabolic :
Negative nitrogen balance because of protein catabolism, fluid and electrolyte disturbances (e.g., sodium and fluid retention, potassium and calcium loss, hypokalaemic alkalosis), antibody production and loss of stimulatory effect of ACTH with prolonged use.
Other :
Infection, musculoskeletal disturbances (e.g., weakness, myopathy, loss of muscle mass, osteoporosis, vertebral compression fractures, pathologic fracture of long bones, aseptic necrosis of femoral and humeral heads), endocrine abnormalities (e.g., menstrual irregularities, growth suppression in children, hirsutism, cushingoid state, glucose intolerance, decreased carbohydrate tolerance, increased requirement for insulin or oral hypoglycaemic agent in diabetic patients, secondary adrenocortical, pituitary unresponsiveness.
EFFECTS ON ABILITY TO DRIVE AND USE MACHINES :
Not known.
INFORMATION FOR PATIENTS :
- Counsel patient to follow dietary regimen carefully (e.g., salt restriction, potassium supplementation).
- Advise patient to avoid receiving live virus vaccinations while taking this medication.
- Instruct patient to have periodic eye examinations while taking medication as long-term therapy.
- If patient has diabetes, instruct to monitor blood glucose regularly throughout therapy since dosage of insulin or oral hypoglycaemic agent may need to be increased.
- Advise patient to contact health care provider before discontinuing medication.
- Instruct patient to notify health care provider at first sign of infection : prolonged cold symptoms, sore throat, weight gain, gastrointestinal upset, heart irregularities, delayed wound healing or changes in mood behaviour.
- Tell patient to report these symptoms to health care
provider : fluid retention, muscle weakness, abdominal pain, seizures, headaches.

 Cardiovascular
Cardiovascular





