100 mg
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
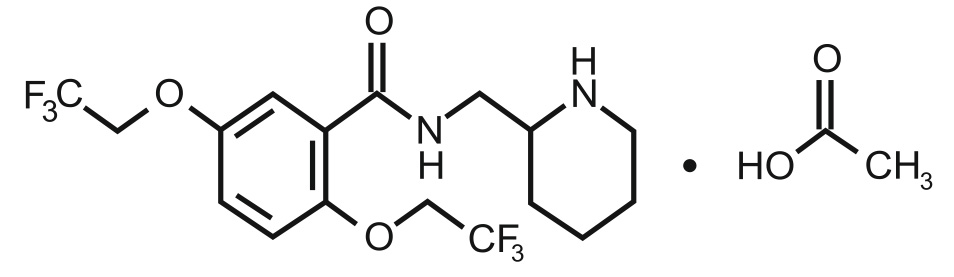
FLECAINIDE ACETATE TABLETS is a class IC antiarrthythmic drug. Chemically, Flecainide acetate is N-(2-Piperidylmethyl)-2,5-bis(2,2,2-trifluoroethoxy)benzamide monoacetate. The molecular formula is C17H20F6N2O3 · C2H4O2 and molecular weight is 474.39.
STRUCTURAL FORMULA :
Its structural formula is :
FLECAINIDE ACETATE TABLETS 50 mg/100 mg/150 mg are white coloured, circular biconvex uncoated tablets with breakline on one side.
COMPOSITION :
Each uncoated tablet contains :
Flecainide Acetate USP 50 mg
Excipients q.s.
Each uncoated tablet contains :
Flecainide Acetate USP 100 mg
Excipients q.s.
Each uncoated tablet contains :
Flecainide Acetate USP 150 mg
Excipients q.s.
ACTIONS :
Flecainide acetate has local anaesthetic activity and belongs to the membrane stabilising (Class I) group of antiarrhythmic agents. It has electro-physiological effects characteristic of the IC (fast inward sodium channel blockers) class of antiarrhythmics. Flecainide Acetate produces a dose related decrease in intracardiac conduction in all parts of the heart with the greatest effect on the His-Purkinje system (H-V conduction). Effects upon atrioventricular (AV) nodal conduction time and intra-atrial conduction times, although present, are less pronounced than those on ventricular conduction velocity. Significant effects on refractory periods are observed in the atria and ventricles. Sinus node recovery times (corrected) following pacing and spontaneous cycle lengths are somewhat increased. This latter effect may become significant in patients with sinus node dysfunction. Flecainide Acetate affects the electrocardiograph (ECG) by widening the PR interval and by prolonging the duration of the QRS complex. The widened QRS complex (ventricular depolarisation) results in a longer QT interval but there is little specific effect on the JT interval (ventricular repolarisation). Flecainide Acetate does not usually alter heart rate, although bradycardia and tachycardia have been reported occasionally. Decreases in ejection fraction, consistent with a negative inotropic effect, have been observed after single dose administration of 200 to 250 mg of flecainide in man in multiple dose studies, and exacerbations of clinical congestive heart failure (CHF) have been documented. Increases in ejection fraction may result from restoring normal rhythm.
PHARMACODYNAMICS :
Supraventricular arrhythmia
In patients with symptomatic paroxysmal atrial fibrillation and flutter, flecainide acetate prolongs the time to the first recurrence as well as the interval between recurrences of these tachyarrhythmias.
Ventricular arrhythmia
Flecainide Acetate causes a dose-related and plasma-level related decrease in single and multiple premature ventricular complexes (PVCs) and chronic therapy can suppress recurrence of ventricular tachycardia. In limited studies of patients with a history of ventricular tachycardia, flecainide has been successful 30 to 40 % of the time in fully suppressing the inducibility of arrhythmias by programmed electrical stimulation.
PHARMACOKINETICS :
Flecainide is almost completely absorbed after oral administration and does not undergo extensive first-pass metabolism. The bioavailability from flecainide acetate tablets has been reported to be about 90 %. Flecainide is extensively metabolised (subject to genetic polymorphism), the 2 major metabolites being m-O-dealkylated flecainide and m-O-dealkylated lactam of flecainide, both of which may have some activity. It is eliminated mainly in the urine, approximately 30 % as unchanged drug and the remainder as metabolites. About 5 % is excreted in the faeces. Excretion of flecainide is decreased in renal failure, heart failure, and in alkaline urine. The therapeutic plasma concentration range is generally accepted as 200 to 1000 ng per ml. The elimination half-life of flecainide is about 20 hours and it is about 40 % bound to plasma proteins. Flecainide passes the placenta and is excreted in breast milk.
INDICATIONS :
FLECAINIDE ACETATE TABLETS are indicated for :
a) AV nodal reciprocating tachycardia; arrhythmias associated with Wolff-Parkinson-White Syndrome and similar conditions with accessory pathways.
b) Paroxysmal atrial fibrillation in patients with disabling symptoms when treatment need has been established and in the absence of left ventricular dysfunction. Arrhythmias of recent onset will respond more readily.
c) Symptomatic sustained ventricular tachycardia.
d) Premature ventricular contractions and/or non-sustained ventricular tachycardia which are causing disabling symptoms, where these are resistant to other therapy or when other treatment has not been tolerated.
FLECAINIDE ACETATE TABLETS can be used for the maintenance of normal rhythm following conversion by other means.
Administration :
FLECAINIDE ACETATE TABLETS is for oral administration.
Dosage :
Adults :
Supraventricular arrhythmias :
The recommended starting dosage is 50 mg twice daily and most patients will be controlled at this dose. If required the dose may be increased to a maximum of 300 mg daily.
Ventricular arrhythmias :
The recommended starting dosage is 100 mg twice daily. The maximum daily dose is 400 mg and this is normally reserved for patients of large build or where rapid control of the arrhythmia is required. After 3 - 5 days it is recommended that the dosage be progressively adjusted to the lowest level which maintains control of the arrhythmia. It may be possible to reduce dosage during long-term treatment.
Elderly patients :
The rate of flecainide elimination from plasma may be reduced in elderly people. This should be taken into consideration when making dose adjustments.
Children :
Flecainide is not recommended in children under 12 years of age as there is insufficient evidence of its use in this age group.
Plasma levels :
Based on PVC suppression, it appears that plasma levels of 200-1000 ng/ml may be needed to obtain the maximum therapeutic effect. Plasma levels above 700-1000 ng/ml are associated with increased likelihood of adverse experiences.
Impaired renal function :
In patients with significant renal impairment (creatinine clearance of 35 ml/min/1.73 sq.m. or less) the maximum initial dosage should be 100 mg daily (or 50 mg twice daily). When used in such patients, frequent plasma level monitoring is strongly recommended.
CONTRAINDICATIONS :
1. Structural heart disease.
2. Second or third degree AV block, unless a ventricular programmable pacemaker is present to sustain rhythm.
3. Right bundle branch block when associated with left hemiblock, unless a pacemaker is utilised to sustain rhythm.
4. Asymptomatic premature ventricular contractions and/or asymptomatic non-sustained ventricular tachycardia in patients with a history of myocardial infarction, cardiogenic shock and reduced cardiac output (LVEF < 35 %). This contraindication may be mitigated in patients with life-threatening ventricular arrhythmias.
5. Cardiogenic shock.
6. Post-myocardial infarction patients.
7. In patients with significant renal or hepatic impairment, unless potential benefits outweigh risks. If used, frequent plasma level monitoring is required to guide dosage.
8. Known hypersensitivity to flecainide or its excipients.
WARNINGS AND PRECAUTIONS :
Electrolyte disturbances should be corrected before using flecainide.
Since flecainide elimination from the plasma can be markedly slower in patients with significant hepatic impairment, flecainide should not be used in such patients unless the potential benefits clearly outweigh the risks. Flecainide should be used with caution in patients with impaired renal function and frequent therapeutic drug monitoring should be undertaken. Plasma level monitoring is strongly recommended in these circumstances. Flecainide is known to increase endocardial pacing thresholds, i.e. to decrease endocardial pacing sensitivity. This effect is reversible and is more marked on the acute pacing threshold than on the chronic. Flecainide should thus be used with caution in all patients with permanent pacemakers or temporary pacing electrodes and should not be administered to patients with existing poor thresholds or non-programmable pacemakers unless suitable pacing rescue is available. Generally, a doubling of either pulse width or voltage is sufficient to regain capture but it may be difficult to obtain ventricular thresholds less than 1 volt at initial implantation in the presence of flecainide.
have pre-existing heart disease with cardiac enlargement, a history of myocardial infarction, arteriosclerotic heart disease and cardiac failure. Flecainide should be avoided in patients with structural organic heart disease or abnormal left ventricular function. Flecainide should be used with caution in patients with acute onset of atrial fibrillation following cardiac surgery. In a large scale, placebo-controlled clinical trial in post-myocardial infraction patients with asymptomatic ventricular arrhythmia, oral flecainide was associated with a 2.2 fold higher incidence of mortality or non-fatal cardiac arrest as compared with its matching placebo. In that same study, an even higher incidence of mortality was observed in flecainide – treated patients with more than one myocardial infraction. Comparable placebo-controlled clinical trials have not been done to determine if flecainide is associated with higher risk of mortality in other patient groups.
Pregnancy : Category C
Adequate and well-controlled studies in humans have not been done.
Studies in New Zealand White rabbits given doses of 30 and 35 mg/kg per day revealed teratogenic and embryotoxic effects. Teratogenic effects did not occur in Dutch Belted rabbits at the same dose or in rats and mice given doses up to 50 and 80 mg/kg per day, respectively. However, delayed sternebral and vertebral ossification was seen at the high dose in rats.
Paediatrics :
Appropriate studies on the relationship of age to the effects of flecainide have not been performed in the paediatric population. Safety and efficacy have not been established.
INTERACTIONS AND INCOMPATIBILITIES :
Flecainide is a class I anti-arrhythmic and interactions are possible with other antiarrhythmic drugs where additive effects may occur or where drugs interfere with the metabolism of flecainide. The following known categories of drugs may interact with flecainide :
Cardiac glycosides :
Flecainide can cause the plasma digoxin level to rise by about 15 %, which is unlikely to be of clinical significance for patients with plasma levels in the therapeutic range. It is recommended that the digoxin plasma level in digitalized patients should be measured not less than 6 hours after any digoxin dose, before or after administration of flecainide.
Class I anti-arrhythmics :
Use of flecainide with other sodium channel blockers (e.g. quinidine) is not recommended. The clearance of flecainide may be decreased by quinidine in patients who are extensive metabolises, since quinidine inhibits the enzyme responsible for the metabolism of flecainide.
Class II anti-arrythmics :
The possibility of additive negative inotropic effects of beta blockers and other cardiac depressants with flecainide should be recognised.
Class III anti-arrythmics :
When flecainide is given in the presence of amiodarone the usual dosage of flecainide should be reduced by 50 % and the patient monitored closely for adverse effects. Plasma level monitoring is strongly recommended in these circumstances.
Class IV anti-arrythmics :
Calcium-channel Blockers: increases myocardial depression and asystole with verapamil.
Other Anti-arrythmics :
Concomitant administration of flecainide with other antiarrhythmics drugs may increase myocardial depression.
Antidepressants :
Fluoxetine increases plasma-flecainide concentration and there is an increased risk of arrhythmias with tricyclics.

 Cardiovascular
Cardiovascular