
5,00,000 IU
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
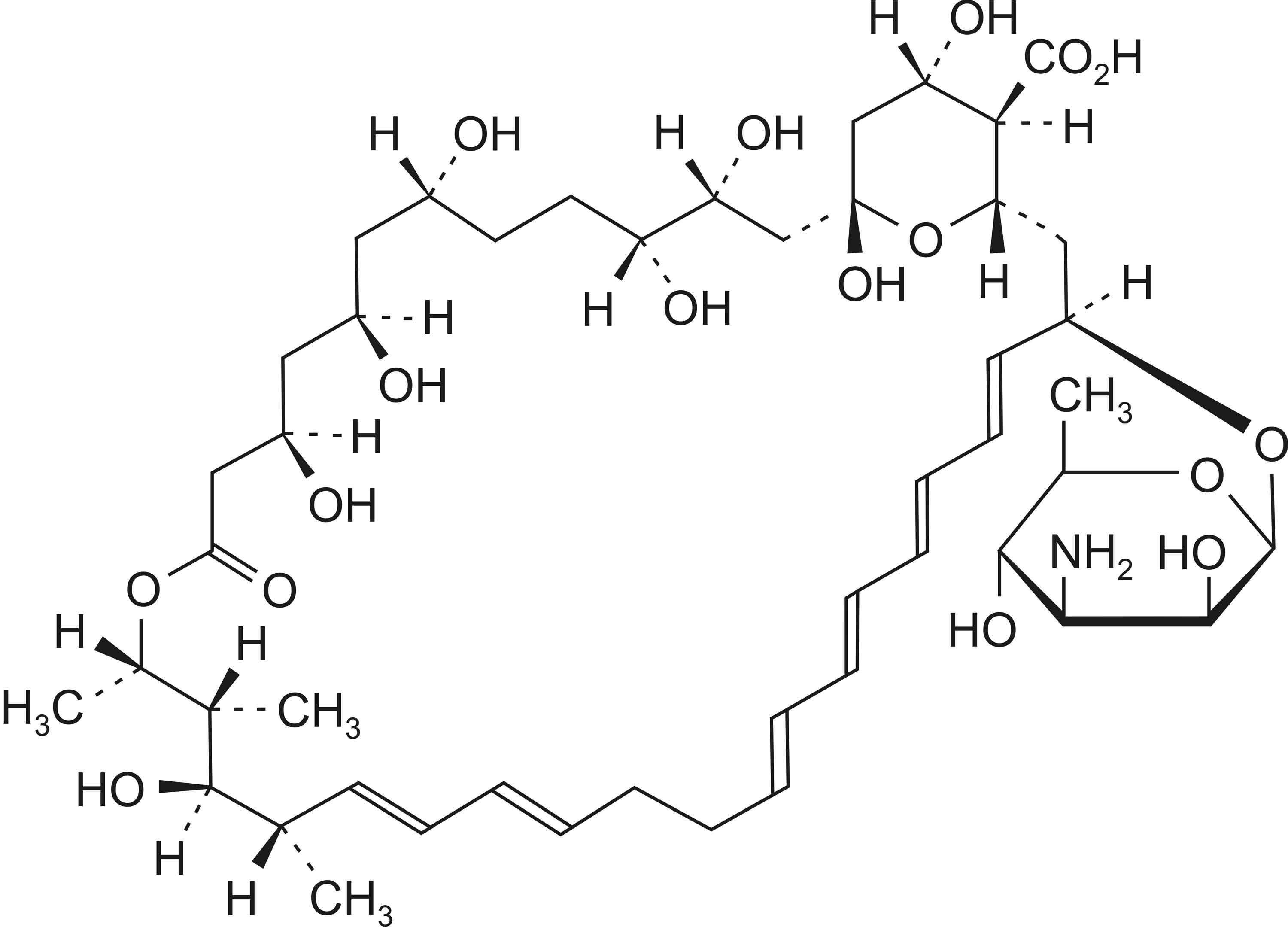
NYSTATIN TABLETS B.P. (Nystatin) is an antifungal antibiotic, produced by a strain of Streptomyces noursei. Chemically, Nystatin is (1S,3R,4R,7R,9R,11R,15S,16R, 17R,18S,19E,21E,25E,27E,29E,31E,33R,35S,36R, 37S)-33-[(3-amino-3,6-dideoxy-β-D-mannopyranosyl)oxy]-1,3,4,7,9,11,17,37-octahydroxy-15,16,18-trimethyl-13-oxo-14,39-dioxabicyclo[33.3.1]nonatriaconta-19,21,25,27,29, 31-hexaene-36-carboxylic acid (nystatin A1). The molecular formula is C47H75NO17 and molecular weight is 926.
STRUCTURAL FORMULA :
Its structural formula is :
NYSTATIN TABLETS B.P. is brown coloured, round film coated tablets.
COMPOSITION :
Each film coated tablet contains :
Nystatin B.P. 5,00,000 IU
Excipients q.s.
Colours : Red Ferric Oxide NF, Yellow Ferric Oxide NF.
Titanium Dioxide B.P.
ACTIONS :
Nystatin is an antifungal antibiotic, produced by a strain of Streptomyces noursei, active against yeasts and yeast like fungi, including Candida albicans. The antifungal activity is probably due to the binding of sterols in the cell membrane of the fungus with a resultant change in membrane permeability allowing leakage of intracellular components. Nystatin has no appreciable activity against bacteria.
PHARMACOKINETICS :
Gastrointestinal absorption of nystatin is insignificant. Most orally administered nystatin is passed unchanged in the stool. In patients with renal insufficiency receiving oral therapy with conventional dosage forms, significant plasma concentrations of nystatin may occasionally occur.
MICROBIOLOGY :
Nystatin is both fungistatic and fungicidal against a wide variety of yeasts and yeast like fungi. Demonstrates no significant resistance to nystatin on repeated subculture in increasing levels of nystatin; other species become quite resistant. Generally, resistance does not develop. Nystatin acts by binding to sterols in the cell membrane of susceptible species with a resultant change in membrane permeability allowing leakage of intracellular components. Nystatin exhibits no appreciable activity against bacteria, protozoa, or viruses. In vitro Candida albicans in vitro Candida in vivo Candida.
INDICATIONS :
NYSTATIN TABLETS B.P. is indicated for the treatment of candidiasis, oral infection and skin infection.
Administration :
NYSTATIN TABLETS B.P. is for oral administration.
Dosage :
For the treatment of intestinal candidiasis :
5,00,000 units every 6 hours, doubled in severe infection.
Neonate :
1,00,000 units 4 times daily.
Child 1 month – 12 years :
1,00,000 units 4 times daily.
Immunocompromised children may require higher doses (e.g. 5,00,000 units 4 times daily).
CONTRAINDICATIONS :
NYSTATIN TABLETS B.P. are contraindicated in patients with a history of hypersensitivity to any of their components. NYSTATIN TABLETS B.P. contains lactose which is contra-indicated in patients with galactosaemia, the glucose-galactose malabsorption syndrome, or lactase deficiency.
PRECAUTIONS :
NYSTATIN TABLETS B.P. is not to be used for the treatment of systemic mycoses. Discontinue treatment if sensitization or irritation is reported during use. NYSTATIN TABLETS B.P. should be used cautiously in diabetic patients.
Pregnancy : Category C
Animal reproduction studies have not been conducted with nystatin. It is also not known whether nystatin can cause foetal harm when administered to a pregnant woman or can affect reproduction capacity. Nystatin should be given to a pregnant woman only if clearly needed.
Nursing mothers :
It is not known whether nystatin is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when nystatin is administered to a nursing woman.
Paediatric Use :
Safety and effectiveness in paediatric patients have not been established.
INTERACTIONS :
None known.
SIDE EFFECTS :
Nystatin is well tolerated even with prolonged therapy. Oral irritation and sensitization have been reported.
Gastrointestinal :
Diarrhoea (including one case of bloody diarrhoea), nausea, vomiting, gastrointestinal upset/disturbances.
Dermatologic :
Rash, including urticaria has been reported rarely. Stevens-Johnson syndrome has been reported very rarely.
Other :
Tachycardia, bronchospasm, facial swelling, and nonspecific myalgia have also been rarely reported.
EFFECTS ON ABILITY TO DRIVE AND USE MACHINES :
None known.
INFORMATION FOR PATIENTS :
The patient should be informed of symptoms of sensitization or irritation and told to report them promptly. The patient should be warned against interruption or discontinuation of medication even though symptomatic relief may occur within a few days.
OVERDOSAGE :
Large oral doses have occasionally produced diarrhoea, gastrointestinal distress, nausea and vomiting. There have been reports of allergic reactions to orally administered Nystatin, although these are rare.
TREATMENT OF OVERDOSAGE :
Treatment should be symptomatic and supportive.
STORAGE :
Store below 30°C (86°F), protected from moisture and light.
Do not refrigerate.
SHELF LIFE :
24 months from the date of manufacture.
PRESENTATION :
NYSTATIN TABLETS B.P. contains Nystatin B.P. 5,00,000 IU.
3 Strips of 10 Tablets per Box.
Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.

 Cardiovascular
Cardiovascular





