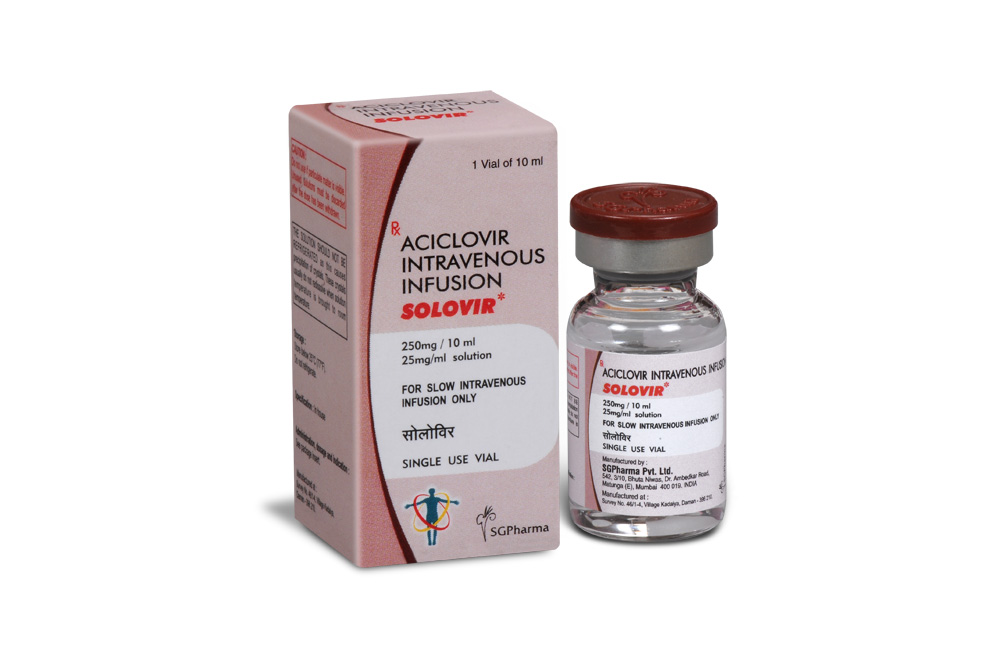
250 mg/10 ml
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
SOLOVIR (Aciclovir) is a synthetic acyclic purine nucleoside analogue. Chemically, Aciclovir is 2-amino-9-[(2-hydroxyethoxy)methyl]-1, 9-dihydro-6H-purin-6-one. Its molecular formula is C8H11N5O3 and molecular weight is 225.2.
STRUCTURAL FORMULA :
Its structural formula is :
-Structure.jpg)
SOLOVIR is a clear colourless to pale yellow, sterile, isotonic solution filled in a vial of suitable size.
COMPOSITION :
Each ml contains :
Aciclovir I.P. 25 mg
Water for Injections I.P. q.s.
Contains no preservatives.
ACTIONS :
Aciclovir is an antiviral agent which is active in vitro against Herpes simplex (HSV) types I and II and Varicella zoster virus (VZV). However, the relationship between in vitro sensitivity of herpes viruses to aciclovir and clinical response to therapy has yet to be established. Aciclovir needs to be phosphorylated to the active compound, aciclovir triphosphate, in order to become active against the virus. Such conversion is very limited in normal cells and in addition cellular DNA polymerase is not very sensitive to the active compound. However, in infected cells HSV or VZV coded thymidine kinases facilitates the conversion of aciclovir to aciclovir monophosphate which is then converted to aciclovir triphosphate by cellular enzymes. Aciclovir triphosphate acts as an inhibitor of, and substrate for, the herpes specified DNA polymerase, preventing further viral DNA synthesis. Animal studies indicate that at high doses aciclovir is cytotoxic.
PHARMACOKINETICS :
In adults, the terminal plasma half-life of aciclovir after the administration of Aciclovir 25 mg/ml Sterile Concentrate is about 3 hours. Aciclovir is widely distributed in tissues and body fluids. Approximately 75-80 % of the drug is excreted unchanged by the kidney. Renal clearance of aciclovir is substantially greater than creatinine clearance, indicating that tubular secretion, in addition to glomerular filtration, contributes to the renal elimination of the drug. 9-carboxymethoxymethylguanine is the major significant metabolite of aciclovir and accounts for 10 to 15 % of the dose excreted in the urine. In adults, mean steady state peak (Cssmax) plasma concentrations following a one-hour infusion were ;
-Table-1.jpg)
In children over 1 year of age similar mean peak (Cssmax) and trough (Cssmin) levels were observed when a dose of 250 mg/m2 was substituted for 5 mg/kg and a dose of 500 mg/m2 was substituted for 10 mg/kg. In neonates (0 to 3 months of age) treated with doses of 10 mg/kg administered by infusion over a one-hour period every 8 hours the Cssmax was found to be 61.2 micromolar (13.8 microgram/ml) and the Cssmin. to be 10.1 micromolar (2.3 microgram/ml). The terminal plasma half-life in neonates was approximately 4 hours. In the elderly, total body clearance falls with increasing age and is associated with decreases in creatinine clearance although there is little change in the terminal plasma half-life.
In patients with end stage renal failure the plasma half-life is increased, extending to a mean terminal half-life of approximately 20 hours. The mean aciclovir half-life during haemodialysis was 5.7 hours. Plasma aciclovir levels dropped approximately 60 % during dialysis.
Cerebrospinal fluid levels are approximately 50 % of corresponding plasma levels. Plasma protein binding is relatively low (9 to 33 %) and drug interactions involving binding site displacement are not anticipated.
INDICATIONS :
SOLOVIR is indicated for the treatment of Herpes simplex infections. SOLOVIR is indicated for the prophylaxis of Herpes simplex infections in immune-compromised patients. SOLOVIR is indicated in the treatment of Varicella zoster infections. SOLOVIR is indicated for the treatment of Herpes simplex infections in the neonate. SOLOVIR formulations are indicated for prophylaxis of CMV infection in bone marrow transplant recipients. It has been shown that high dose intravenous aciclovir reduces the incidence and delays the onset of CMV infection. When high dose intravenous aciclovir is followed by 6 months treatment with high dose oral aciclovir mortality and the incidence of viraemia are also reduced.
Administration :
The required dose of SOLOVIR should be administered by slow intravenous infusion over a one-hour period. From the calculated dose, determine the appropriate number of vials to be used. To reconstitute each vial, add the recommended volume of infusion fluid and shake gently until the contents of the vial have dissolved completely. After reconstitution SOLOVIR may be administered by a controlled-rate infusion pump. Alternatively, the reconstituted solution may be further diluted to give an aciclovir concentration of not greater
than 5 mg/ml (0.5 % w/v) for administration by infusion. Add the required volume of reconstituted solution to the chosen infusion solution, as recommended below, and shake well to ensure adequate mixing occurs. For children and neonates, where it is advisable to keep
the volume of infusion fluid to a minimum, it is recommended that dilution is on the basis of 4 ml reconstituted solution (100 mg aciclovir) added to 20 ml of infusion fluid.
For adults, it is recommended that infusion bags containing 100 ml of infusion fluid are used, even when this would give an aciclovir concentration substantially below 0.5 % w/v. Thus one 100 ml infusion bag may be used for any dose between 250 mg and 500 mg aciclovir (10 and 20 ml of reconstituted solution) but a second bag must be used for doses between 500 and 1000 mg. When diluted in accordance with the recommended schedules, SOLOVIR is known to be compatible with the following infusion fluids and stable for up to 12 hours at room temperature (15°C to 25°C).
• Sodium Chloride Intravenous Infusion B.P. (0.45 % and 0.9 % w/v);
• Sodium Chloride (0.18 % w/v) and Glucose (4 % w/v) Intravenous Infusion B.P.;
• Sodium Chloride (0.45 % w/v) and Glucose (2.5 % w/v) Intravenous Infusion B.P.;
• Compound Sodium Lactate Intravenous Infusion B.P. (Hartmanns Solution).
SOLOVIR when diluted in accordance with the above schedule will give an aciclovir concentration not greater than 0.5 % w/v. Since no antimicrobial preservative is included, reconstitution and dilution must be carried out under full aseptic conditions, immediately before use, and any unused solution discarded. Should any visible turbidity or crystallisation appear in the solution before or during infusion, the preparation should be discarded.
Dosage :
Dosage in adults :
Patients with Herpes simplex (except Herpes encephalitis) or Varicella zoster infections should be given SOLOVIR in doses of 5 mg/kg bodyweight every 8 hours. Immune-compromised patients with Varicella zoster infections or patients with Herpes encephalitis should be given SOLOVIR in doses of 10 mg/kg bodyweight every 8 hours provided renal function is not impaired. For prophylaxis of CMV infection in bone marrow transplant recipients 500 mg/m2 aciclovir should be given intravenously 3 times daily at approximately 8 hourly intervals. The duration of treatment recommended in bone marrow transplant recipients is from 5 days before up to 30 days after transplant.
Dosage in children :
The dose of SOLOVIR for children aged between 3 months and 12 years is calculated on the basis of body surface area. Children with Herpes simplex (except Herpes encephalitis) or Varicella zoster infections should be given SOLOVIR in doses of 250 mg per square metre body surface area every 8 hours. In immune-compromised children with Varicella zoster infections or children with Herpes encephalitis, SOLOVIR should be given in doses of 500 mg per square metre body surface area every 8 hours if renal function is not impaired. Limited data suggest that for the prophylaxis of CMV infection in children, over 2 years of age, who have undergone bone marrow transplantation, the adult dose may be given. Children with impaired renal function require an appropriately modified dose, according to the degree of impairment.
Dosage in Neonates :
The dosage of SOLOVIR in neonates is calculated on the basis of bodyweight. Neonates with Herpes simplex infections should be given SOLOVIR in doses of 10 mg/kg bodyweight every 8 hours.
Dosage in the Elderly :
In the elderly total aciclovir body clearance declines in parallel with creatinine clearance. Special attention should be given to dosage reduction in elderly patients with impaired creatinine clearance.
Dosage in Renal Impairment :
Caution is advised when administering SOLOVIR to patients with impaired renal function. The following adjustments in dosage are suggested. A course of treatment with SOLOVIR usually lasts 5 days, but this may be adjusted according to the patients condition and response to therapy. Treatment for Herpes encephalitis and neonatal Herpes simplex infections usually lasts 10 days. The duration of prophylactic administration of SOLOVIR is determined by the duration of the period at risk.
CONTRAINDICATIONS :
SOLOVIR is contraindicated in patients known to be previously hypersensitive to aciclovir and valaciclovir.
WARNINGS :|
SOLOVIR is intended for intravenous infusion only and should not be used by any other route. SOLOVIR has a pH of approximately 11.0 and should not be administered by mouth. SOLOVIR must be given over a period of at least one hour in order to avoid renal tubular damage. It should not be administered as a bolus injection. Although the aqueous solubility of aciclovir sodium (for infusion) exceeds 100mg/ml, precipitation of aciclovir crystals in renal tubules, and the consequent renal tubular damage, can occur if the maximum solubility of free aciclovir
(2.5 mg/mL at 37°C in water) is exceeded. Aciclovir infusion must be accompanied by adequate hydration. Since maximum urine concentration occurs within the first few hours following infusion, particular attention should be given to establish sufficient urine flow during that period. Concomitant use of other nephrotoxic drugs, pre-existing renal disease and dehydration increase the risk of further renal impairment by aciclovir. As aciclovir has been associated with reversible encephalopathic changes, it should be used with caution in patients with underlying neurological abnormalities, significant hypoxia or serious renal, hepatic or electrolyte abnormalities. It should also be used with caution in patients who have manifested neurological reactions to cytotoxic drugs or are receiving concomitantly interferon or intrathecal methotrexate. Resistant strains have been isolated in vitro and in animals following treatment with aciclovir. HSV strains resistant in vitro to aciclovir have also been isolated from immunocompromised patients receiving aciclovir for Herpes simplex infections. Therefore the potential for the development of resistant HSV strains in the patients treated with aciclovir should be borne in mind. The relationship between in vitro sensitivity of herpes viruses to aciclovir and clinical response to therapy has yet to be established.
In patients receiving Aciclovir 25 mg/ml Sterile Concentrate at higher doses (e.g. for Herpes encephalitis), specific care regarding renal function should be taken, particularly when patients are dehydrated or have any renal impairment. Contact with eyes or unprotected skin should be avoided. Solovir contains sodium metabisulphite, a sulphite that may cause allergic-type reactions including anaphylactic symptoms and life-threatening or less severe asthmatic episodes in certain susceptible people. The overall prevalence of sulphite sensitivity in the general population is unknown and probably low. Sulphite sensitivity is seen more frequently in asthmatic than non-asthmatic people.
PRECAUTIONS :
Precipitation of aciclovir crystals in renal tubules can occur if the maximum solubility of free aciclovir (2.5 mg/ml at 37°C in water) is exceeded or if the drug is administered by bolus injection. Ensuing renal tubular damage can produce acute renal failure. Abnormal renal function (decreased creatinine clearance) can occur as a result of aciclovir administration and depends on the state of the patients hydration, other treatments, and the rate of drug administration. Concomitant use of other nephrotoxic drugs, pre-existing renal disease, and dehydration make further renal impairment with aciclovir more likely. Administration of aciclovir injection by intravenous infusion must be accompanied by adequate hydration. When dosage adjustments are required, they should be based on estimated creatinine clearance.
Approximately 1 % of patients receiving intravenous aciclovir have manifested encephalopathic changes characterized by either lethargy, obtundation, tremors, confusion, hallucinations, agitation, seizures, or coma. Aciclovir injection should be used with caution in those patients who have underlying neurologic abnormalities and those with serious renal, hepatic, or electrolyte abnormalities, or significant hypoxia.
Pregnancy :
Limited data are available on the use of aciclovir during pregnancy. Caution should therefore be exercised by balancing the potential benefits of treatment against any possible hazard.
Lactation :
Following oral administration of 200 mg five times a day, aciclovir has been detected in human breast milk at concentrations ranging from 0.6 to 4.1 times the corresponding plasma levels. These levels would potentially expose nursing infants to aciclovir dosages of up to
0.3 mg/kg bodyweight/day. Caution is therefore advised if aciclovir is to be administered to a nursing woman.
INTERACTIONS :
Aciclovir is eliminated primarily unchanged in the urine via renal tubular secretion. Any drugs administered concurrently that compete with this mechanism or affect renal physiology may increase aciclovir plasma concentration. Probenecid and cimetidine increases the aciclovir mean half-life and area under the plasma concentration curve. Increases in plasma AUCs of aciclovir and of the inactive metabolite of mycophenolate mofetil, an immunosuppressant agent used in transplant patients, have been shown when the drugs are coadministered. Care is also required (with monitoring for changes in renal function) if administering intravenous aciclovir with drugs that affect other aspects of renal physiology (e.g. cyclosporin, tacrolimus). In patients over 60 years of age concurrent use of diuretics increases plasma levels of aciclovir very significantly. It is not known whether a similar effect occurs in young adults. In patients receiving zidovudine no significant overall increase in toxicity was associated with the addition of aciclovir. No data are available on interactions between aciclovir and other antiretroviral therapies. Aciclovir should be used with caution in patients who have manifested neurological reactions to cytotoxic drugs or are receiving concomitantly interferon or intrathecal methotrexate.
SIDE EFFECTS :
LocaI :
Local inflammation or phlebitis at injection site.
Systemic :
Renal : Rapid increases in blood urea and creatinine levels may occur occasionally in patients given SOLOVIR. These are usually reversible but progression to acute renal failure can occur in rare cases. The risk of renal damage is increased by bolus injection, dehydration, concomitant use of other nephrotoxic drugs and pre-existing renal disease. To avoid this effect the drug should not be given as an intravenous bolus injection but by slow infusion over a one hour period. Hypersensitivity and Skin : Rashes including
photosensitivity, urticaria, pruritis, fevers and rarely dyspnoea, angiodema and anaphylaxis. Neurological : Approximately 1 % of patients receiving aciclovir have manifested encephalopathic changes characterised by one or more of the following: lethargy, obtundation, tremors, confusion, hallucinations, agitation, somnolence, psychosis, seizures, and coma.
Haematological : Decrease in haematological indices (anaemia, thrombocytopenia, leucopenia).
Gastrointestinal : Nausea and vomiting.
Liver : Reversible increases in bilirubin and liver related enzymes. Hepatitis and jaundice have been reported on very rare occasions.
Others : Less frequent adverse effects include diaphoresis, haematuria, hypotension and headache.
OVERDOSAGE :
Overdosage of intravenous aciclovir has resulted in elevations of serum creatinine, blood urea nitrogen and subsequent renal failure. Neurological effects including confusion, hallucinations, agitation, seizures and coma have been described in association with overdosage.
TREATMENT OF OVERDOSAGE :
Adequate hydration is essential to reduce the possibility of crystal formation in the urine. Aciclovir can be removed from circulation by haemodialysis and this may therefore be considered an option in the management of overdose of this drug.
PHARMACEUTICAL PRECAUTIONS :
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. SOLOVIR is an antiviral agent. When handling, a laboratory coat and latex gloves are adequate protection. Where solution accidentally contacts the eye or skin, the affected area should be rinsed thoroughly. Medical attention should be sought in the event of eye contamination. Following accidental swallowing, the mouth should be rinsed out with water ensuring that the mouth wash is not swallowed and the person should be given about 250 ml of water to drink. Vomiting should not be induced, but medical attention should be sought. It is unlikely that first aid would be required as a result of inhalation during normal use.
STORAGE :
Store below 30°C (86°F).
Do not refrigerate.
SHELF LIFE :
24 months from the date of manufacture.
PRESENTATION :
SOLOVIR is supplied as below :
-Table-2.jpg)
Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.

 Cardiovascular
Cardiovascular