150 mg
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
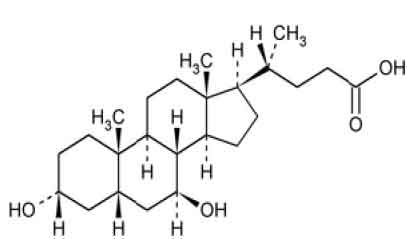
URSODIOL TABLETS (Ursodiol) is a naturally occurring bile acid present in small quantities in human bile. It is a anticholelithic. Chemically, Ursodiol is 3α,7β-Dihydroxy-5β- cholan-24-oic acid. The molecular formula is C24H40O4 and molecular weight is 392.57.
STRUCTURAL FORMULA :
Its structural formula is :
URSODIOL TABLETS are brown coloured, circular, biconvex film coated tablets plain on both side.
COMPOSITION :|
Each film coated tablet contains :
Ursodiol USP 150 mg
Excipients q.s.
Colours : Red Ferric Oxide NF, Yellow Ferric Oxide NF
ACTIONS :
Although the exact mechanism of ursodiol’s anticholelithic action is not completely understood, it is known that when administered orally ursodiol is concentrated in bile and decreases biliary cholesterol saturation by suppressing hepatic synthesis and secretion of cholesterol, and by inhibiting its intestinal absorption. The reduced cholesterol saturation permits the gradual solubilization of cholesterol from gallstones, resulting in their eventual dissolution.
Other actions/effects :
Ursodiol increases bile flow. In chronic cholestatic liver disease, ursodiol appears to reduce the detergent properties of the bile salts, thus reducing their cytotoxicity. Also, ursodiol may protect liver cells from the damaging activity of toxic bile acids (e.g., lithocholate,
deoxycholate, and chenodeoxycholate), which increase in concentration in patients with chronic liver disease.
PHARMACOKINETICS :
Ursodiol is normally present as a minor fraction of the total bile acids in humans (about 5 %). Following oral administration, the majority of ursodiol is absorbed by passive diffusion and its absorption is incomplete. Once absorbed, ursodiol undergoes hepatic extraction to the extent of about 50 % in the absence of liver disease. As the severity of liver disease increases, the extent of extraction decreases. In the liver, ursodiol is conjugated with glycine or taurine, then secreted into bile. These conjugates of ursodiol are absorbed in the small intestine by passive and active mechanisms. The conjugates can also be deconjugated in the ileum by intestinal enzymes, leading to the formation of free ursodiol that can be reabsorbed and reconjugated in the liver. Nonabsorbed ursodiol passes into the colon where it is mostly 7-dehydroxylated to lithocholic acid. Some ursodiol is epimerized to chenodiol (CDCA) via a 7-oxo intermediate. Chenodiol also undergoes 7-dehydroxylation to form lithocholic acid. These metabolites are poorly soluble and excreted in the faeces. A small portion of lithocholic acid is reabsorbed, conjugated in the liver with glycine, or taurine and sulfated at the 3 position. The resulting sulfated lithocholic acid conjugates are excreted in bile and then lost in faeces.
In healthy subjects, at least 70 % of ursodiol (unconjugated) is bound to plasma protein. No information is available on the binding of conjugated ursodiol to plasma protein in healthy subjects or PBC patients. Its volume of distribution has not been determined, but is expected to be small since the drug is mostly distributed in the bile and small intestine. Ursodiol is excreted primarily in the faeces. With treatment, urinary excretion increases, but remains less than 1 % except in severe cholestatic liver disease. During chronic administration of ursodiol, it becomes a major biliary and plasma bile acid. At a chronic dose of 13 to 15 mg/kg/day, ursodiol constitutes 30 - 50 % of biliary and plasma bile acids.
INDICATIONS :
URSODIOL TABLETS are indicated in the treatment of primary biliary cirrhosis (PBC) and for the dissolution of small to medium sized radiolucent, cholesterol-rich gall-stones in patients with a functioning gall bladder. Cholesterol stones coated with calcium or stones composed of bile pigments are not dissolved by ursodiol. Ursodiol has a particular place in the treatment of patients in whom surgery is contraindicated or who are anxious to avoid surgery.
Administration :
URSODIOL TABLETS are for oral administration.
URSODIOL TABLETS should be taken with a drink of water, meals or snack since it dissolves more rapidly when bile and pancreatic juice are present in the intestinal chyme.
Dosage :
Primary Biliary Cirrhosis :
Adults and Elderly : 10 - 15 mg ursodiol per kg per day in two to four divided doses.
Children : Dosage should be related to body weight.
Dissolution of gallstones : The usual dose is 6 - 12 mg/kg/day either as a single night time dose or in 2 or 3 divided doses. This may be increased to 15 mg/kg/day in obese patients, if necessary.
The duration of treatment may be up to two years, depending on the size of the stone(s), and should be continued for three months after the apparent dissolution of the stone(s). A dose of 300 mg twice daily has been suggested for the prevention of gallstones in patients undergoing rapid weight loss. Ursodiol has also been given in reduced doses in combination with chenodeoxycholic acid.
CONTRAINDICATIONS :
- Acute inflammation of the gallbladder or biliary tract.
- Occlusion of the biliary tract (occlusion of the common bile duct or a cystic duct).
- Frequent episodes of biliary colic.
- Radio-opaque calcified gallstones.
- Impaired contractility of the gallbladder.
- Non functioning gall bladder.
- Hypersensitivity to bile acids or to any of the excipients in the formulation.
- Inflammatory bowel disease.
- Hepatic and intestinal conditions interfering with enterohepatic recirculation of bile acids :
- Extrahepatic cholestasis.
- Intrahepatic cholestasis.
- Ileal resection.
- Regional ileitis.
- Ileal stoma.
- Acute, chronic or severe liver disease.
- Active duodenal ulcer.
- Active gastric ulcer.
WARNINGS AND PRECAUTIONS :
URSODIOL TABLETS should be taken under medical supervision. During the first 3 months of treatment, the liver function parameters AST (SGOT), ALT (SGPT) and γ-GT should be monitored by the physician every 4 weeks, thereafter every 3 months. Apart from allowing for identification of responders and non-responders in patients being treated for primary biliary cirrhosis, this monitoring would also enable early detection of potential hepatic deterioration, particularly in patients with advanced stage primary biliary cirrhosis.
When used for the dissolution of cholesterol gallstones :
In order to assess therapeutic progress and for timely detection of any calcification of the gallstones, depending on stone size, the gall bladder should be visualised (oral cholecystography) with overview and occlusion views in standing and supine positions (ultrasound control) 6 - 10 months after the beginning of treatment. If the gall bladder cannot be visualised on X-ray images, or in cases of calcified gallstones, impaired contractility of the gall bladder or frequent episodes of biliary colic, ursodiol should not be used.
When used for treatment of advanced stage of primary biliary cirrhosis :
In very rare cases decompensation of hepatic cirrhosis has been observed, which partially regressed after the treatment was discontinued. If diarrhoea occurs, the dose must be reduced and in cases of persistent diarrhoea, the therapy should be discontinued. Excessive dietary intake of calories and cholesterol should be avoided ; a low cholesterol diet will probably improve the effectiveness of URSODIOL TABLETS.
Pregnancy : Category B
Reproduction studies have been performed in pregnant rats at oral doses up to 22 times the recommended maximum human dose (based on body surface area) and in pregnant rabbits at oral doses up to 7 times the recommended maximum human dose (based on body surface area) and have revealed no evidence of impaired fertility or harm to the foetus due to ursodiol. There are no adequate or well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug
should be used during pregnancy only if clearly needed.
Nursing mothers :
It is not known whether ursodiol is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when URSODIOL TABLETS are administered to a nursing mother.
Paediatric Use :
The safety and effectiveness of URSODIOL TABLETS in paediatric patients have not been established.
EFFECTS ON ABILITY TO DRIVE AND USE MACHINES :
No effects on the ability to drive and use machines have been observed.
INTERACTIONS :
URSODIOL TABLETS should not be administered concomitantly with charcoal, colestyramine, colestipol or antacids containing aluminium hydroxide and/or smectite (aluminium oxide), because these preparations bind ursodiol in the intestine and thereby inhibit its
absorption and efficacy. Should the use of a preparation containing one of these substances be necessary, it must be taken at least 2 hours before or after ursodiol. Ursodiol can increase the absorption of cyclosporine from the intestine. In patients receiving cyclosporine treatment, blood concentrations of this substance should therefore be checked by the physician and the cyclosporine dose adjusted if necessary. In isolated cases ursodiol can reduce the absorption of ciprofloxacin.
Ursodiol has been shown to reduce the plasma peak concentrations (Cmax) and the area under the curve (AUC) of the calcium antagonist nitrendipine. An interaction with a reduction of the therapeutic effect of dapsone was also reported. These observations together with in vitro findings could indicate a potential for ursodiol to induce cytochrome P450 3A enzymes. Controlled clinical trials have shown, however, that ursodiol does not have a relevant inductive effect on cytochrome P450 3A enzymes. Oral contraceptives, oestrogenic hormones and blood cholesterol lowering agents such as clofibrate may increase biliary lithiasis, which is a counter - effect to ursodiol used for dissolution of gallstones.
SIDE EFFECTS :
URSODIOL TABLETS is normally well tolerated. No significant alterations have so far been observed in liver function.
OVERDOSAGE :
Signs and symptoms :
Diarrhoea may occur. In general, other symptoms of overdose are unlikely because the absorption of Ursodiol decreases with increasing dose and therefore more is excreted with the faeces.
TREATMENT OF OVERDOSAGE :
No specific counter-measures are necessary and the consequences of diarrhoea should be treated symptomatically with restoration of fluid and electrolyte balance. However, ion-exchange resins may be useful to bind bile acids in the intestine. Liver function tests monitoring is recommended.
STORAGE :
Store below 25°C, protected from moisture and light.
Do not refrigerate.
SHELF LIFE :
24 months from the date of manufacture.
PRESENTATION :
URSODIOL TABLETS contain Ursodiol USP 150 mg.
3 Blisters of 10 Tablets per Box.
Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.

 Cardiovascular
Cardiovascular