0.500 mg/ml, 5 mg/5 ml
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
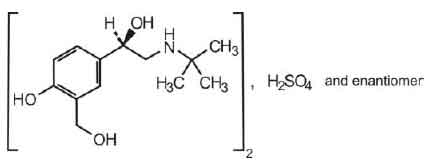
SALBUTAMOL INJECTION (Salbutamol) is used as bronchodilator in the management of asthma and chronic obstructive pulmonary disease. Chemically, Salbutamol sulphate is bis [(1RS-2-[1,1-dimethylethyl)amino]-1-4-hydroxy-3-ydroxymethyl)phenyl]ethanol]sulphate.The
molecular formula is (C13H21NO3)2H2SO4 and molecular weight is 576.7.
STRUCTURAL FORMULA :
Its structural formula is :
SALBUTAMOL INJECTION is a colourless or very pale yellow solution filled in amber ampoule of suitable size.
COMPOSITION :
Each ml contains :
Salbutamol Sulphate B.P.
equivalent to Salbutamol 0.500 mg
Water for Injections B.P. q.s.
ACTIONS :
Salbutamol is a bronchodilator act by stimulating Beta2 – adrenergic receptors in the lungs to relax bronchial smooth muscle, thereby relieving bronchospasm. This action is believed to result from increased production of cyclic adenosine 3,5-monophosphate (cyclic 3,5 –AMP ; cAMP and ensuing reduction in intracellular calcium concentration caused by activation of the enzyme adenylate cyclase that catalyzes the conversion of adenosine triphosphate (ATP) to cAMP. Increased cAMP concentrations, in addition to relaxing bronchial smooth muscle, inhibit release of mediators of immediate hypersensitivity from cells, especially from mast cells.
PHARMACOKINETICS :
Salbutamol administered intravenously has a half-life of 4 to 6 hours and is cleared partly renally and partly by metabolism to the inactive 4’ – O-sulphate (phenolic sulphate) which is also excreted primarily in the urine. The faeces are a minor route of excretion. The majority of a dose of Salbutamol given intravenously, orally or by inhalation is excreted within 72 hours. Salbutamol is bound to plasma protein to the extent of 10 %
INDICATIONS :
SALBUTAMOL INJECTION is used for the prevention and treatment of reversible bronchospasm associated with asthma and other obstructive pulmonary diseases. Adjunctive treatment of hyperkalaemia in patients undergoing dialysis.
Administration :

Pregnancy :
Although adequate and well-controlled studies in pregnant women have not been done with inhaled adrenergic bronchodilators, Salbutamol, is used in pregnancy when any potential risk that may be associated with treatment is preferable to the risk of placental hypoxaemia from uncontrolled pulmonary disease. Extensive use of adrenergic bronchodilators during pregnancy has provided no evidence that the sympathomimetic class effects seen in animal studies are relevant to human use.
Nursing Mothers :
As salbutamol is probably secreted in breast milk its use in nursing mothers is not recommended unless the expected benefits outweigh any potential risk. In such situations the use of the inhaled route may be preferable although it is not known whether salbutamol has a harmful effect on the neonate.
Paediatric Use :
Use of Salbutamol, in children has not demonstrated paediatrics – specific problems that would limit their usefulness.
INTERACTIONS AND INCOMPATIBILITES :
Beta – Blockers : Severe bronchospasms may be produced in asthmatic patients taking Salbutamol.
Digoxin : Salbutamol may decrease serum digoxin levels.
Diuretics : ECG changes and hypocalcaemia associated with these diuretics may worsen with Salbutamol co administration.
SIDE EFFECTS :
ide effects are listed below by system organ class and frequency. Frequencies are defined as : very common (≥1/10), common (≥1/100 and <1/10), uncommon (≥1/1000 and <1/100), rare (≥ 1/10,000 and <1/1000) and very rare (<1/10,000) Including isolated reports. Very common and common events were generally etermined from clinical trial data. Rare and very rare events were generally determined from spontaneous data.
Immune system disorders
Very rare : Hypersensitivity reactions including angioedema, urticaria, bronchospasm, hypotension and collapse.
Metabolism and nutrition disorders
Rare : Hypocalcaemia. Potentially serious hypocalcaemia may result from beta2 agonist therapy.
Very rare : Lactic acidosis Lactic acidosis has been reported very rarely in patients receiving intravenous and nebulised Salbutamol therapy for the treatment of acute asthma exacerbation.
Nervous system disorders
Very common : Tremor.
Common : Headache.
Very rare : Hyperactivity.
Cardiac disorders
Very common : Tachycardia, palpitations.
Uncommon : Myocardial ischaemia
In the management of pre – term labour
Rare : Cardiac arrhythmias including atrial fibrillation, supraventricular tachycardia and extrasystoles.
Vascular disorders
Rare : Peripheral vasodilatation.
Respiratory, thoracic and mediastinal disorders
Uncommon : Pulmonary oedema.
In the management of pre-term labour, salbutamol Injection/ solution for infusion have uncommonly been associated with pulmonary oedema. Patients with predisposing factors including multiple pregnancies, fluid overload, maternal infection and pre-eclampsia may have an increased risk of developing pulmonary oedema.
Gastrointestinal disorders
Very rare : Nausea, vomiting In the management of premature labour, intravenous infusion of Salbutamol Injection has very rarely been associated with nausea and vomiting. Musculoskeletal and connective tissue disorders Common : Muscle cramps. Injury, poisoning and procedural complications Very rare : Slight pain or stinging on I.M. use of undiluted injection.
OVERDOSAGES AND TREATMENT OF OVERDOSAGES:
The most common signs and symptoms of overdose with salbutamol are transient beta agonist pharmacologically mediated events, including tachycardia, tremor, hyperactivity and metabolic effects including hypocalcaemia and lactic acidosis. Hypocalcaemia may occur following overdose with salbutamol. Serum potassium levels should be monitored. Nausea, vomiting and hyperglycaemia have been reported, predominantly in children and when salbutamol overdose has been taken via the oral route. Consideration should be given to discontinuation of treatment and appropriate symptomatic therapy such as cardio-selective beta-blocking agents in patients presenting with cardiac symptoms (e.g. tachycardia, palpitations). Beta-blocking drugs should be used with caution in patients with a history of bronchospasm.
PHARMACEUTICAL PRECAUTIONS :
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
STORAGE :
Store below 30ºC, protected from light.
Do not refrigerate.
SHELF LIFE :
24 months from the date of manufacture.
PRESENTATION :
SALBUTAMOL INJECTION contains Salbutamol Sulphate B.P. equivalent to Salbutamol 0.500 mg in 1 ml aqueous solution.
Such 10 Ampoules are packed in box.
Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.

 Cardiovascular
Cardiovascular