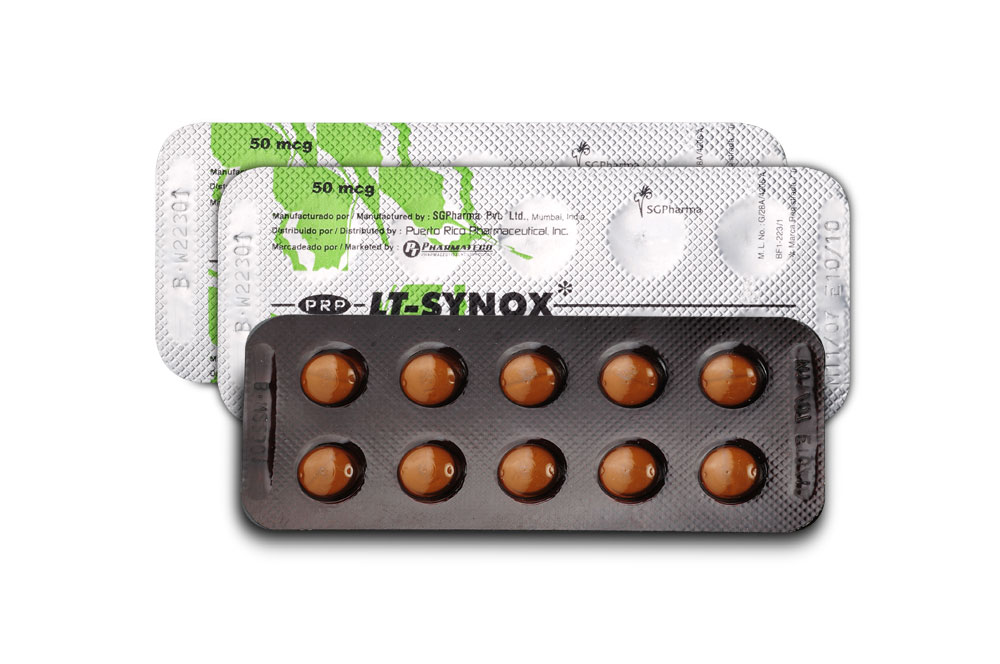
50 mcg
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
LT-SYNOX (Liothyronine Sodium) contains (L-triiodothyronine or LT3), a synthetic form of a natural thyroid hormone. Chemically, liothyronine sodium is L-Tyrosine, O-(4-hydroxy-3-iodophenyl)-3,5-diiodo-, monosodium salt. The molecular formula is C15H11I3NNaO4 and the molecular weight is 672.96.
STRUCTURAL FORMULA :
Its structural formula is :
-Structure.jpg)
LT - SYNOX 5 mcg tablets are white in colour, round, beveled, scored and debossed with PRP on one side.
LT - SYNOX 25 mcg tablets are white in colour, round, beveled, scored and debossed with PRP on one side.
LT - SYNOX 50 mcg tablets are white in colour, round, beveled, scored and debossed with PRP on one side.
COMPOSITION :
LT - SYNOX 5 mcg
Each tablet contains :
Liothyronine Sodium USP
equivalent to Liothyronine 5 mcg
Excipients q.s.
LT - SYNOX 25 mcg
Each tablet contains :
Liothyronine Sodium USP
equivalent to Liothyronine 25 mcg
Excipients q.s.
LT - SYNOX 50 mcg
Each tablet contains:
Liothyronine Sodium USP
equivalent to Liothyronine 50 mcg
Excipients q.s.
ACTIONS :
The mechanisms by which thyroid hormones exert their physiologic action are not well understood. These hormones enhance oxygen consumption by most tissues of the body, increase the basal metabolic rate and the metabolism of carbohydrates, lipids and proteins. Thus, they exert a profound influence on every organ system in the body and are of particular importance in the development of the central nervous system.
PHARMACOKINETICS :
Since liothyronine sodium (T3) is not firmly bound to serum protein, it is readily available to body tissues. The onset of activity of liothyronine sodium is rapid, occurring within a few hours. Maximum pharmacologic response occurs within 2 or 3 days providing early clinical response. The biological half-life is about 2 ½ days. T3 is almost totally absorbed, 95 % in 4 hours. The hormones contained in the natural preparations are absorbed in a manner similar to the synthetic hormones. Liothyronine sodium has a rapid cutoff of activity which permits quick dosage adjustment and facilitates control of the effects of overdosage, should they occur. The higher affinity of levothyroxine (T4) for both thyroid-binding globulin and thyroid-binding prealbumin as compared to triiodothyronine (T3) partially explains the higher serum levels and longer half-life of the former hormone. Both protein-bound hormones exist in reverse equilibrium with minute amounts of free hormone, the latter accounting for the metabolic activity.
INDICATIONS :
Thyroid hormone drugs are indicated :
1. As replacement or supplemental therapy in patients with hypothyroidism of any etiology, except transient hypothyroidism during the recovery phase of subacute thyroiditis. This category includes cretinism, myxedema and ordinary hypothyroidism in patients of any age (paediatric patients, adults, the elderly), or state (including pregnancy); primary hypothyroidism resulting from functional deficiency, primary atrophy, partial or total absence of thyroid gland, or the effects of surgery, radiation, or drugs, with or without the presence of goiter and secondary (pituitary) or tertiary (hypothalamic) hypothyroidism.
2. As pituitary thyroid-stimulating hormone (TSH) suppressants, in the treatment or prevention of various types of euthyroid goiters, including thyroid nodules, subacute or chronic lymphocytic thyroiditis (Hashimotos) and multinodular goiter.
3. As diagnostic agents in suppression tests to differentiate suspected mild hyperthyroidism or thyroid gland autonomy.
LT - SYNOX tablets can be used in patients allergic to desiccated thyroid or thyroid extract derived from pork or beef.
LT - SYNOX tablets are intended for oral administration.
Dosage :
The dosage of thyroid hormones is determined by the indication and must in every case be individualized according to patient response and laboratory findings. Once-a-day dosage is recommended. Although liothyronine sodium has a rapid cutoff, its metabolic effects persist for a few days following discontinuance.
Mild Hypothyroidism :
Recommended starting dosage is 25 mcg daily. Daily dosage then may be increased by up to 25 mcg every 1 or 2 weeks. Usual maintenance dose is 25 to 75 mcg daily. The rapid onset and dissipation of action of liothyronine sodium (T3), as compared with levothyroxine sodium (T4), has led some clinicians to prefer its use in patients who might be more susceptible to the untoward effects of thyroid medication. However, the wide swings in serum T3 levels that follow its administration and the possibility of more pronounced cardiovascular side effects tend to counterbalance the stated advantages. LT - SYNOX tablets may be used in preference to levothyroxine (T4) during radioisotope scanning procedures, since induction of hypothyroidism in those cases is more abrupt and can be of shorter duration. It may also be preferred when impairment of peripheral conversion of T4 to T3 is suspected.
Myxedema :
Recommended starting dosage is 5 mcg daily. This may be increased by 5 to 10 mcg daily every 1 or 2 weeks. When 25 mcg daily is reached, dosage may be increased by 5 to 25 mcg every 1 or 2 weeks until a satisfactory therapeutic response is attained. Usual maintenance dose is 50 to 100 mcg daily.
Myxedema Coma :
Myxedema coma is usually precipitated in the hypothyroid patient of long standing by intercurrent illness or drugs such as sedatives and anaesthetics and should be considered a medical emergency. An intravenous preparation of liothyronine sodium may be used in myxedema coma / precoma.
Congenital Hypothyroidism :
Recommended starting dosage is 5 mcg daily, with a 5 mcg increment every 3 to 4 days until the desired response is achieved. Infants a few months old may require only 20 mcg daily for maintenance. At 1 year, 50 mcg daily may be required. Above 3 years, full adult dosage may be necessary.
Simple (non-toxic) Goiter :
Recommended starting dosage is 5 mcg daily. This dosage may be increased by 5 to 10 mcg daily every 1 or 2 weeks. When 25 mcg daily is reached, dosage may be increased every week or two by 12.5 or 25 mcg. Usual maintenance dosage is 75 mcg daily. In the elderly or in paediatric patients, therapy should be started with 5 mcg daily and increased only by 5 mcg increments at the recommended intervals. When switching a patient to LT - SYNOX Tablets from thyroid, L-thyroxine or thyroglobulin, discontinue the other medication, initiate LT - SYNOX tablets at a low dosage, and increase gradually according to the patients response. When selecting a starting dosage, bear in mind that this drug has a rapid onset of action and that residual effects of the other thyroid preparation may
persist for the first several weeks of therapy.
Thyroid Suppression Therapy :
Administration of thyroid hormone in doses higher than those produced physiologically by the gland results in suppression of the production of endogenous hormone. This is the basis for the thyroid suppression test and is used as an aid in the diagnosis of patients with signs of mild hyperthyroidism in whom baseline laboratory tests appear normal or to demonstrate thyroid gland autonomy in patients with Graves ophthalmopathy. 131I uptake is determined before and after the administration of the exogenous hormone. A 50 % or greater suppression of uptake indicates a normal thyroid-pituitary axis and thus rules out thyroid gland autonomy. LT - SYNOX tablets are given in doses of 75 to 100 mcg/day for 7 days and radioactive iodine uptake is determined before and after administration of the hormone. If thyroid function is under normal control, the radioiodine uptake will drop significantly after treatment. LT - SYNOX tablets should be administered cautiously to patients in whom there is a strong suspicion of thyroid gland autonomy, in view of the fact that the exogenous hormone effects will be additive to the endogenous source.
CONTRAINDICATIONS :
Thyroid hormone preparations are generally contraindicated in patients with diagnosed but as yet uncorrected adrenal cortical insufficiency, untreated thyrotoxicosis and apparent hypersensitivity to any of their active or extraneous constituents. There is no well-documented evidence from the literature, however, of true allergic or idiosyncratic reactions to thyroid hormone.
WARNINGS :
Drugs with thyroid hormone activity, alone or together with other therapeutic agents, have been used for the treatment of obesity. In euthyroid patients, doses within the range of daily hormonal requirements are ineffective for weight reduction. Larger doses may produce serious or even life-threatening manifestations of toxicity, particularly when given in association with sympathomimetic amines such as those used for their anorectic effects. The use of thyroid hormones in the therapy of obesity, alone or combined with other drugs, is unjustified and has been shown to be ineffective. Neither is their use justified for the treatment of male or female infertility unless this condition is accompanied by hypothyroidism.
Thyroid hormones should be used with great caution in a number of circumstances where the integrity of the cardiovascular system, particularly the coronary arteries, is suspected. These include patients with angina pectoris or the elderly, in whom there is a greater likelihood of occult cardiac disease. In these patients, liothyronine sodium therapy should be initiated with low doses, with due consideration for its relatively rapid onset of action. Starting dosage of LT - SYNOX tablets is 5 mcg daily, and should be increased by no more than 5 mcg increments at 2-week intervals. When, in such patients, a euthyroid state can only be reached at the expense of an aggravation of the cardiovascular disease, thyroid hormone dosage should be reduced. Morphologic hypogonadism and nephrosis should be ruled out before the drug is administered. If hypopituitarism is present, the adrenal deficiency must be corrected prior to starting the drug. Myxedematous patients are very sensitive to thyroid; dosage should be started at a very low level and increased gradually.
Severe and prolonged hypothyroidism can lead to a decreased level of adrenocortical activity commensurate with the lowered metabolic state. When thyroid-replacement therapy is administered, the metabolism increases at a greater rate than adrenocortical activity. This can precipitate adrenocortical insufficiency. Therefore, in severe and prolonged hypothyroidism, supplemental adrenocortical steroids may be necessary. In rare instances the administration of thyroid hormone may precipitate a hyperthyroid state or may aggravate
existing hyperthyroidism.
PRECAUTIONS :
General :
Thyroid hormone therapy in patients with concomitant diabetes mellitus or insipidus or adrenal cortical insufficiency aggravates the intensity of their symptoms. Appropriate adjustments of the various therapeutic measures directed at these concomitant endocrine diseases are required. The therapy of myxedema coma requires simultaneous administration of glucocorticoids. Hypothyroidism decreases and hyperthyroidism increases the sensitivity to oral anticoagulants. Prothrombin time should be closely monitored in thyroidtreated patients on oral anticoagulants and dosage of the latter agents adjusted on the basis of frequent prothrombin time determinations. In infants, excessive doses of thyroid hormone preparations may produce craniosynostosis.
Pregnancy : Category A.
Thyroid hormones do not readily cross the placental barrier. The clinical experience to date does not indicate any adverse effect on foetuses when thyroid hormones are administered to pregnant women. On the basis of current knowledge, thyroid replacement therapy to hypothyroid women should not be discontinued during pregnancy.
Lactation :
Minimal amounts of thyroid hormones are excreted in human milk. Thyroid is not associated with serious adverse reactions and does not have a known tumorigenic potential. However, caution should be exercised when thyroid is administered to a nursing woman.
Paediatric Use :
Pregnant mothers provide little or no thyroid hormone to the foetus. The incidence of congenital hypothyroidism is relatively high (1:4000) and the hypothyroid foetus would not derive any benefit from the small amounts of hormone crossing the placental barrier. Routine determinations of serum T4 and/or TSH is strongly advised in neonates in view of the deleterious effects of thyroid deficiency on growth and development. Treatment should be initiated immediately upon diagnosis and maintained for life, unless transient hypothyroidism is suspected, in which case, therapy may be interrupted for 2 to 8 weeks after the age 3 years to reassess the condition. Cessation of therapy is justified in patients who have maintained a normal TSH during those 2 to 8 weeks.
INFORMATION FOR PATIENTS :
Patients on thyroid hormone preparations and parents of paediatric patients on thyroid therapy should be informed that :
1. Replacement therapy is to be taken essentially for life, with the exception of cases of transient hypothyroidism, usually associated with thyroiditis, and in those patients receiving a therapeutic trial of the drug.
2. They should immediately report during the course of therapy any signs or symptoms of thyroid hormone toxicity, e.g. chest pain, increased pulse rate, palpitations, excessive sweating, heat intolerance, nervousness or any other unusual event.
3. In case of concomitant diabetes mellitus, the daily dosage of antidiabetic medication may need readjustment as thyroid hormone replacement is achieved. If thyroid medication is stopped, a downward readjustment of the dosage of insulin or oral hypoglycemic agent may be necessary to avoid hypoglycemia. At all times, close monitoring of urinary glucose levels is mandatory in such patients.
4. In case of concomitant oral anticoagulant therapy, the prothrombin time should be measured frequently to determine if the dosage of oral anticoagulants is to be readjusted.
5. Partial loss of hair may be experienced by paediatric patients in the first few months of thyroid therapy, but this is usually a transient phenomenon and later recovery is usually the rule.
Laboratory Tests :
Treatment of patients with thyroid hormones requires the periodic assessment of thyroid status by means of appropriate laboratory tests besides the full clinical evaluation. The TSH suppression test can be used to test the effectiveness of any thyroid preparation, bearing in mind the relative insensitivity of the infant pituitary to the negative feedback effect of thyroid hormones. Serum T4 levels can be used to test the effectiveness of all thyroid medications except products containing liothyronine sodium. When the total serum T4 is low but TSH is normal, a test specific to assess unbound (free) T4 levels is warranted. Specific measurements of T4 and T3 by competitive protein binding or radioimmunoassay are not influenced by blood levels of organic or inorganic iodine and have essentially replaced older tests of thyroid hormone measurements, i.e. PBI, BEI and T4 by column.
INTERACTIONS AND INCOMPATIBILITIES :
Oral Anticoagulants :
Thyroid hormones appear to increase catabolism of vitamin K-dependent clotting factors. If oral anticoagulants are also being given, compensatory increases in clotting factor synthesis are impaired. Patients stabilized on oral anticoagulants that are found to require thyroid replacement therapy should be watched very closely when thyroid is started. If a patient is truly hypothyroid, it is likely that a reduction in anticoagulant dosage will be required. No special precautions appear to be necessary when oral anticoagulant therapy is begun in a patient already stabilized on maintenance thyroid replacement therapy.
Insulin or Oral Hypoglycaemics :
Initiating thyroid replacement therapy may cause increases in insulin or oral hypoglycaemic requirements. The effects seen are poorly understood and depend upon a variety of factors such as dose and type of thyroid preparations and endocrine status of the patient. Patients receiving insulin or oral hypoglycaemics should be closely watched during initiation of thyroid replacement therapy.
Cholestyramine :
Cholestyramine binds both T4 and T3 in the intestine, thus impairing absorption of these thyroid hormones. In vitro studies indicate that the binding is not easily removed. Therefore, 4 to 5 hours should elapse between administration of cholestyramine and thyroid hormones.
Oestrogen, Oral Contraceptives :
Oestrogens tend to increase serum thyroxine-binding globulin (TBg). In a patient with a nonfunctioning thyroid gland who is receiving thyroid replacement therapy, free levothyroxine may be decreased when oestrogens are started thus increasing thyroid requirements. However, if the patients thyroid gland has sufficient function, the decreased free thyroxine will result in a compensatory increase in thyroxine output by the thyroid. Therefore, patients without a functioning thyroid gland who are on thyroid replacement therapy may need to increase their thyroid dose if oestrogens or oestrogen-containing oral contraceptives are given.
Tricyclic Antidepressants :
Use of thyroid products with imipramine and other tricyclic antidepressants may increase receptor sensitivity and enhance antidepressant activity; transient cardiac arrhythmias have been observed. Thyroid hormone activity may also be enhanced.
Digitalis :
Thyroid preparations may potentiate the toxic effects of digitalis. Thyroid hormonal replacement increases metabolic rate, which requires an increase in digitalis dosage.
Ketamine :
When administered to patients on a thyroid preparation, this parenteral anaesthetic may cause hypertension and tachycardia. Use with caution and be prepared to treat hypertension, if necessary.
Vasopressors :
Thyroxine increases the adrenergic effect of catecholamines such as epinephrine and norepinephrine. Therefore, injection of these agents into patients receiving thyroid preparations increases the risk of precipitating coronary insufficiency, especially in patients with coronary artery disease. Careful observation is required.
Drug / Laboratory Test Interactions :
The following drugs or moieties are known to interfere with laboratory tests performed in patients on thyroid hormone therapy : androgens, corticosteroids, estrogens, oral contraceptives containing estrogens, iodine-containing preparations and the numerous preparations containing salicylates.
1. Changes in TBg concentration should be taken into consideration in the interpretation of T4 and T3 values. In such cases, the unbound (free) hormone should be measured. Pregnancy, estrogens and estrogencontaining oral contraceptives increase TBg concentrations. TBg may also be increased during infectious hepatitis. Decreases in TBg concentrations are observed in nephrosis, acromegaly and after androgen or corticosteroid therapy. Familial hyper- or hypo-thyroxine-bindingglobulinemias have been described. The incidence of TBg deficiency approximates 1 in 9000. The binding of thyroxine by thyroxine-binding prealbumin (TBPA) is inhibited by salicylates.
2. Medicinal or dietary iodine interferes with all in vivo tests of radioiodine uptake, producing low uptakes which may not be reflective of a true decrease in hormone synthesis.
3. The persistence of clinical and laboratory evidence of hypothyroidism inspite of adequate dosage replacement indicates either poor patient compliance, poor absorption, excessive faecal loss, or inactivity of the preparation. Intracellular resistance to thyroid hormone is quite rare.
SIDE EFFECTS :
The following effects are indicative of excessive dosage and usually disappear on reduction of doasge or withdrawal of treatment for a day or two. Anginal pain, cardiac arrhythmias, palpitation and cramps in skeletal muscle; also tachycardia, diarrhoea, restlessness, excitability, headache, flushing, sweating, excessive loss of weight and muscular weakness.
OVERDOSAGE :
Signs and Symptoms :
Headache, irritability, nervousness, sweating, arrhythmia (including tachycardia), increased bowel motility and menstrual irregularities. Angina pectoris or congestive heart failure may be induced or aggravated. Shock may also develop. Massive overdosage may result in symptoms resembling thyroid storm. Chronic excessive dosage will produce the signs and symptoms of hyperthyroidism.
TREATMENT OF OVERDOSAGE :
Dosage should be reduced or therapy temporarily discontinued if signs and symptoms of overdosage appear. Treatment may be reinstituted at a lower dosage. In normal individuals, normal hypothalamic-pituitary-thyroid axis function is restored in 6 to 8 weeks after thyroid suppression. Treatment of acute massive thyroid hormone overdosage is aimed at reducing gastrointestinal absorption of the drugs and counteracting central and peripheral effects, mainly those of increased sympathetic activity. Vomiting may be induced initially if further gastrointestinal absorption can reasonably be prevented and barring contraindications such as coma, convulsions or loss of the gagging reflex. Treatment is symptomatic and supportive. Oxygen may be administered and ventilation maintained. Cardiac glycosides may be indicated if congestive heart failure develops. Measures to control fever, hypoglycaemia or fluid loss should be instituted if needed. Antiadrenergic agents, particularly propranolol, have been used advantageously in the treatment of increased sympathetic activity. Propranolol may be administered intravenously at a dosage of 1 to 3 mg over a 10-minute period or orally, 80 to 160 mg/day, especially when no contraindications exist for its use.
STORAGE :
Store at controlled room temperature 15 - 30°C (59 - 86°F), protected from moisture and light.
Do not refrigerate.
SHELF LIFE :
36 months from the date of manufacture.
PRESENTATION :
LT - SYNOX tablets contains Liothyronine Sodium USP equivalent to Liothyronine 5 mcg.
LT - SYNOX tablets contains Liothyronine Sodium USP equivalent to Liothyronine 25 mcg.
LT - SYNOX tablets contains Liothyronine Sodium USP equivalent to Liothyronine 50 mcg.
3 blisters of 10 tablets per box.
For the use of Registered Medical Practitioners or a Hospital or an Institution only.
Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.
 Cardiovascular
Cardiovascular