500 mg/5 ml, 600 mg/3 ml
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
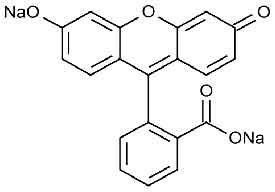
FLUORESCEIN INJECTION USP (Fluorescein Sodium) is a diagnostic dye. Chemically, Fluorescein Sodium is Spiro[isobenzofuran-1(3H),9’-[9H]xanthene]-3-one,3’6’-dihydroxy, disodium salt. Its molecular formula is C20H10Na2O5 and molecular weight is 376.27.
STRUCTURAL FORMULA :
Its structural formula is :
FLUORESCEIN INJECTION USP is a sterile, clear, dark reddish orange solution filled in amber ampoule of suitable size.
COMPOSITION :
Each ml contains :
Fluorescein Sodium USP 100 mg
Water for Injection USP q.s.
Each ml contains :
Fluorescein Sodium USP 200 mg
Water for Injection USP q.s.
ACTIONS :
Fluorescein sodium responds to electromagnetic radiation and light between the wavelengths of 465-490 nm and fluoresces, i.e., emits light at wavelengths of 520-530 nm. Thus, the hydrocarbon is excited by blue light and emits light that appears yellowish-green. Following intravenous injection of fluorescein sodium in an aqueous solution, the unbound fraction of the fluorescein can be excited with a blue light flash from a fundus camera as it circulates through the ocular vasculature, and the yellowish green fluorescence of the dye is captured by the camera. In the fundus, the fluorescence of the dye demarcates the retinal and/or choroidal vasculature under observation, distinguishing it from adjacent areas/structures.
PHARMACOKINETICS :
Absorption
After intravenous injection, fluorescein is rapidly distributed throughout the body and appears in the retinal tissues within a few seconds. After 15 min of intravenous administration, concentration of fluorescein glucuronide, metabolite of fluorescein which also has fluorescent properties, was higher when compared to fluorescein. Intravenous administration of 188 mg fluorescein sodium resulted in Cmax of 10.9 μg/ml and AUC 1350 μg.min/ml. Fluorescein appears in the central artery of the eye, within 7 to 14 seconds after intravenous administration into the antecubital vein. The mean peak concentration for the 10 % fluorescein sodium solution in the retinal artery is amounted to 0.5 mg/ml.
Distribution
Fluorescein binds to albumin and red blood cells in a reversible fashion and the binding is moderate (~70-80 %) during the first hour. About 15-17 % is bound to erythrocytes. Within a few minutes of intravenous administration of fluorescein sodium, a yellowish discolouration of the skin occurs, which begins to fade after 6 to 12 hours of dosing. Various estimates of volume of distribution indicate that fluorescein distributes well into interstitial space (0.5 to 0.8 l/kg).
Metabolism
Fluorescein undergoes rapid metabolism to fluorescein monoglucuronide. After intravenous administration of fluorescein sodium (14 mg/kg) to 7 healthy subjects, approximately 80 % of fluorescein in plasma was converted to glucuronide after a period of 1 hour post-dose, indicating relatively rapid conjugation. Fluorescein monoglucuronide is about 1/3 to 1/4 as fluorescent as fluorescein, depending on the wavelength of excitation of the blue light. Terminal half-lives of fluorescein and fluorescein glucuronide in plasma are approximately 23.5 and 264 minutes, respectively. The glucuronide contributes almost all the plasma fluorescence after 4 to 5 hours. Fluorescein glucuronide is less bound to plasma than fluorescein. Diabetic and non-diabetic patients demonstrate similar fluorescein pharmacokinetics in the plasma.
Excretion
Fluorescein and its metabolites are mainly eliminated via renal excretion. After intravenous administration, the urine remains slightly fluorescent for 24 to 36 hours. A renal clearance of 1.75 ml/min/kg and a hepatic clearance (due to conjugation) of 1.50 ml/min/kg have been estimated. The systemic clearance of fluorescein was essentially complete by 48 to 72 hours after administration of 500 mg fluorescein.
Special populations
Renal impairment :
The plasma concentrations of free fluorescein and fluorescein glucuronide had been elevated in patients with renal insufficiency. Systemic fluorescein did not have influence in glomerular filtration rate in patients with chronic kidney disease, thus dose adjustments in patients with renal impairment are not warranted.
Hepatic impairment :
There is no study information on pharmacokinetics of fluorescein in patients with impaired hepatic function.
INDICATIONS :
FLUORESCEIN INJECTION USP is indicated for the Fluorescein angiography or angioscopy of the fundus and of the iris vasculature. This medicinal product is for diagnostic use only.
Administration :
FLUORESCEIN INJECTION USP is administered Intravenously. Do not mix or dilute with other solutions or drugs. Flush intravenous cannulas before and after drugs are injected to avoid physical incompatibility reactions.
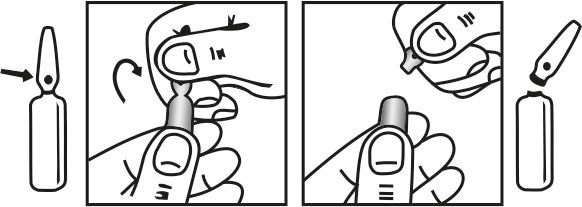
INSTRUCTIONS FOR USE OF AMPOULE :
The ampoule used in this product is equipped with O.P.C (One Point Cut) opening system. No ampoule file is needed to open the ampoule. The neck of the ampoule is prescored at the point of constriction. A coloured dot on the ampoule head helps to orientate the ampoule. Take the ampoule and face the coloured dot. Let the solution at the head of the ampoule to flow down by shaking or a gentle stroke. The ampoule opens easily by placing the thumb on the coloured dot and gently pressing downwards as shown.
Dosage :
The normal adult dose of FLUORESCEIN INJECTION USP is 500 mg (100 mg/ml) via intravenous administration. For children, the dose should be calculated on the basis of 35 mg for each ten pounds of body weight (7.7 mg/kg body weight). Inject the dose rapidly (over 5-10 seconds is normally recommended) into the antecubital vein, after taking precautions to avoid extravasation. A syringe, filled with FLUORESCEIN INJECTION USP , may be attached to transparent tubing and a 23 gauge butterfly needle for injection. Insert the needle and draw the patient’s blood to the hub of the syringe so that a small air bubble separates the patient’s blood in the tubing from the fluorescein. With the room lights on, slowly inject the blood back into the vein while watching the skin over the needle tip. If the needle has extravasated, the patient’s blood will be seen to bulge the skin and the injection should be stopped before any fluorescein is injected. When assured that extravasation has not occurred, the room light may be turned off and the fluorescein injection completed. Luminescence usually appears in the retina and choroidal vessels in 7 to 14 seconds and can be observed by standard viewing equipment. Reduction in dose from 500 mg to 200 mg of FLUORESCEIN INJECTION USP may be appropriate in cases when a highly sensitive imaging system e.g., scanning laser ophthalmoscope is used.
CONTRAINDICATIONS :
Hypersensitivity to the active substance or to any of the excipients. FLUORESCEIN INJECTION USP should not be injected intrathecally or intra-arterial.
PRECAUTIONS :
NOT FOR INTRATHECAL USE – FOR OPHTHALMIC DIAGNOSTIC USE ONLY
Hypersensitivity Reactions :
Hypersensitivity reactions, including rare cases of anaphylactic/anaphylactoid shock (some with fatal outcome), have been reported in patients receiving Fluorescein. If serious hypersensitivity reactions have occurred during previous angiography with other diagnostic agents or there is a history of severe allergic reactions, the need for fluorescein angiography must be very carefully considered and the diagnostic importance balanced against the risk of a severe, possibly fatal (rate 1 in 220,000 angiographies as collected in a survey), allergic reaction.
Managing risk of hypersensitivity reaction with fluorescein angiography requires :
• The patient must be kept under close observation for at least 30 minutes after angiography.
• A protocol for management of anaphylaxis, and an emergency tray with appropriate resuscitation equipment such as adrenaline for intravenous or intramuscular use, intravenous fluids, oxygen, volume substitution and corticosteroids, should always be available in case of such reaction.
Cardiovascular Disease :
Before administration, a complete medical history must be obtained, including history of allergy, history of cardio-pulmonary disease, concomitant medication (in particular beta blockers, including eye drops). Patients with a history of cardiovascular disease require careful evaluation before undergoing an elective procedure with sodium fluorescein. Rarely, severe cardiovascular complications such as chest pain, myocardial infarction and death have occurred following administration of fluorescein sodium. Caution is to be exerted in patients with a history of allergy or bronchial asthma.
Combination with Beta-blockers :
Combination with beta-blockers may in rare cases cause lethal anaphylactic reactions. In patients identified as being at risk of hypersensitivity reactions, but in whom a fluorescein angiography is considered to be essential, the procedure must be carried out in the presence of a specialist in resuscitation, particularly when the patient is under beta-blocker therapy, including beta-blocker eye-drops, as they may require more intensive resuscitation measures due to reduced efficacy of adrenaline and volume expansion.
Extravasation :
Care must be taken to avoid extravasation during injection. The high pH of the fluorescein solution can result in severe local tissue damage. Complications from extravasation can cause severe pain, thrombophlebitis and an inflammatory reaction of the tissue leading to tissue necrosis. Before fluorescein is administered, precautions to avoid extravasation must be taken and the correct intravenous position of the needle tip must be ascertained. In case extravasation occurs, the injection must be stopped immediately and appropriate measures must be taken to treat damaged tissue and to relieve pain.
Special Instructions :
The skin and urine may be coloured yellow but this is transient. Fluorescein sodium can stain skin, clothing, and soft contact lenses on contact.
Carcinogenicity and genotoxicity :
No carcinogenicity studies are available. A standard set of in vitro and in vivo genotoxicity studies were negative (Ames bacterial mutagenicity, chromosomal aberration in CHO cells and chromosome aberration and micronucleus tests in mouse bone marrow). Positive results were obtained in a mouse lymphoma cell thymidine kinase assay as well as in sister chromatid exchange studies in vitro (CHO cells) and in vivo (mouse bone marrow cells). Using a weight of evidence approach for the evaluation of the data summarized above, it is concluded that fluorescein has no clinically relevant genotoxic potential. Intravenous administration of fluorescein sodium did not produce embryotoxic and teratogenic effects in rats or rabbits at doses as high as 1,000, 500, and 31.3 mg/kg, respectively (approximately 100-, 50-, and 3.1- times the recommended human dose).
Effect on Laboratory Tests :
The fluorescence may interfere with the analysis of blood and urinary parameters. Systemic concentration of fluorescein had been reported to interfere in the determination of digoxin and cortisol in serum when fluorescence polarization immunoassay based analysers are used. Caution is advised when performing therapeutic drug monitoring for drugs with a narrow therapeutic window, e.g. digoxin, quinidine.
Effects on Ability to Drive and Use Machines :
No effects of Fluorescein that would adversely influence the ability to drive are known. However, the mydriasis and cycloplegia required for the fluorescein angiography examination may affect vision. Patients should be advised that this might impair their ability to drive or to operate machinery.
Pregnancy : Category C.
Teratogenic Effects :
Adequate animal reproduction studies have not been conducted with fluorescein sodium. It is also not known whether fluorescein sodium can cause foetal harm when administered to a pregnant woman. Fluorescein sodium should be given to a pregnant woman only if clearly needed.
Lactation :
Fluorescein sodium has been demonstrated to be excreted in human milk. Caution should be exercised when fluorescein sodium is administered to a nursing woman.
Paediatric use :
Safety and effectiveness in paediatric patients have been established.
INTERACTIONS :
Fluorescein is a relatively inert dye and specific drug interaction studies have not been reported. There are few case reports on potential interactions with organic anion transporters and interference with certain laboratory tests. Compounds that inhibit or compete with the active transport of organic anions (e.g., probenicid) may affect the systemic profile of fluorescein. The concomitant use of FLUORESCEIN INJECTION USP with beta-blocking agents (including eye-drops solutions) may rarely provoke severe anaphylactic reactions.
Concomitant intravenous injection of other solutions or the mixing of FLUORESCEIN INJECTION USP with other solutions should be avoided as the possibility of interactions cannot be excluded. It is possible that fluorescein may influence certain blood and urine values for 3 to 4 days after application.
INCOMPATIBILITIES :
This medicinal product must not be mixed with other medicinal products. Drugs with acidic pH (especially antihistamines,e.g. promethazine) or citric acid may lead to precipitation of fluorescein and should not be given simultaneously through the intravenous line.
SIDE EFFECTS :
The most frequently reported treatment related undesirable effects were nausea, vomiting, syncope and pruritis. More severe adverse reactions have been reported shortly after fluorescein injection such as angioedema, respiratory disorders (bronchospasm, laryngeal oedema, respiratory failure), anaphylactic shock, hypotension, respiratory arrest and cardiac arrest. Additionally, a yellowish discoloration of the skin could appear but usually disappears within 6 to 12 hours. Urine, which may also exhibit a bright yellow colouration, returns to its normal colour after 24 to 36 hours. The following undesirable effects were assessed to be treatment-related and are classified according to the following convention : very common (≥1/10), common (>1/100 to <1/10), uncommon (>1/1000 to ≤1/100), rare (>1/10,000 to ≤1/1000), or very rare (≤1/10,000). Within each frequency grouping, undesirable effects are presented in decreasing order of seriousness.
OVERDOSAGE :
No specific measures are known.
TREATMENT OF OVERDOSAGE :
In case of overdose with clinical signs, general supportive treatment should be provided.
PHARMACEUTICAL PRECAUTIONS :
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
STORAGE :
Store below 30 oC (86 oF), protected from light.
Do not refrigerate.
SHELF LIFE :
24 months from the date of manufacture.
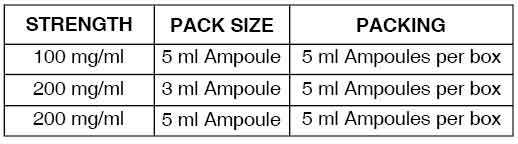
PRESENTATION :
FLUORESCEIN INJECTION USP is supplied as :
Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.
 Cardiovascular
Cardiovascular