600 mg
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
MUCOTYLE (Acetylcysteine) is the N-Acetyl derivative of the naturally occurring amino acid, L-cysteine. Chemically, Acetylcysteine is designated as N-acetyl-L-cysteine. Its molecular formula is C5H9NO3S and its molecular weight is 163.20.
STRUCTURAL FORMULA :
Its structural formula is :
-Structure.jpg)
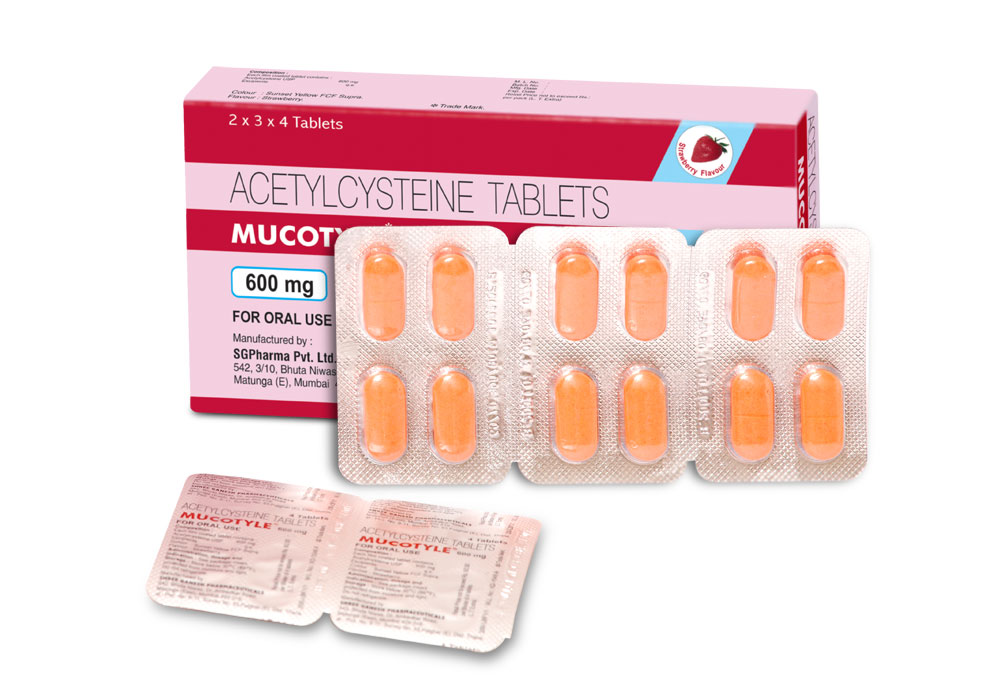
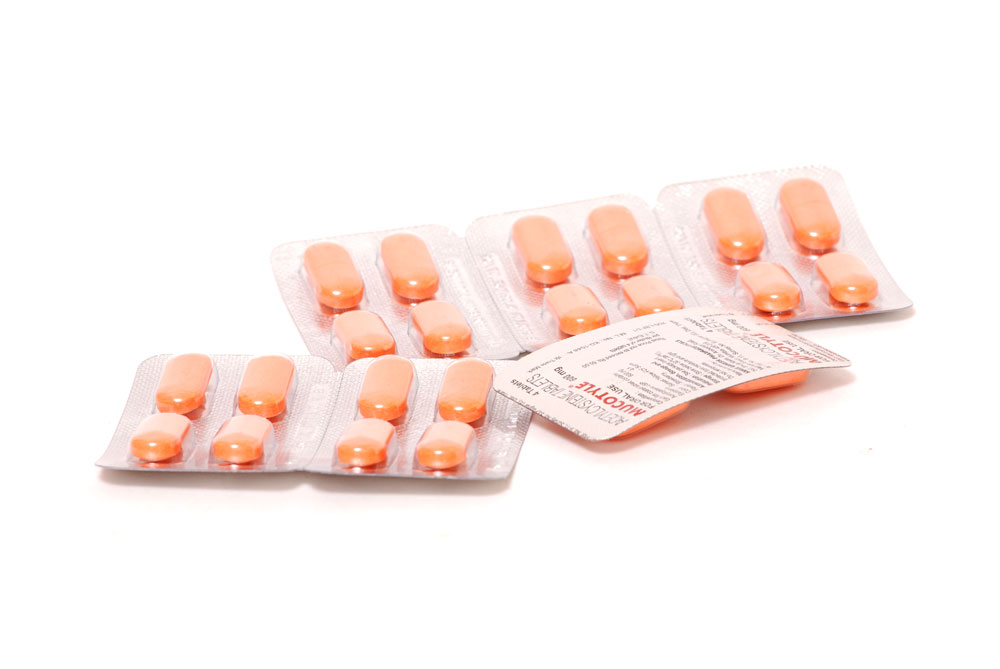
MUCOTYLE Tablets are orange coloured strawberry flavoured film coated tablets with breakline on one side.
COMPOSITION :
Each film coated tablet contains :
Acetylcysteine USP............................... 600 mg
Excipients ............................................ q.s.
Colour : Sunset Yellow FCF Supra.
Flavour : Strawberry.
ACTIONS :
MUCOLYTIC :
Acetylcysteine Tablet contains N-Acetylcysteine, a mucolytic agent. It reduces the viscosity of bronchial secretions. The free sulphydryl group in Acetylcysteine Tablet breaks the disulphide bridges present in the mucus & thereby causes mucolysis. Further, in the mucus producing cells, Acetylcysteine Tablet prevents the formation of disulphide bonds & thereby regulates the viscosity of the mucus. Also, as a precursor of glutathione, an endogenous antioxidant, Acetylcysteine Tablet ensures a protective action on the respiratory system. Thus, it not only protects the respiratory function, but also improves it.
ACETAMINOPHEN POISONING :
Paracetamol is metabolised in the liver, mainly by conjugation with glucuronide and sulphate. It is also metabolised by cytochrome P450 to form a reactive, potentially toxic, metabolite. This metabolite is normally detoxified by conjugation with hepatic glutathione, to form non-toxic derivatives. In paracetamol overdosage, the glucuronide and sulphate conjugation pathways are saturated, so that more of the toxic metabolite is formed. As hepatic glutathione stores are depleted, this toxic metabolite may bind to hepatocyte proteins, leading to liver cell damage and necrosis. Acetylcysteine is a sulphydryl (SH) group donor and may protect the liver from damage by restoring depleted hepatic-reduced glutathione levels, or by acting as an alternative substrate for conjugation with and thus detoxification of, the toxic paracetamol metabolite.
PHARMACOKINETICS :
Acetylcysteine is the N-Acetyl derivative of the naturally occurring amino acid, L-cysteine and is deacetylated in the liver to cysteine or oxidised to other metabolites such as N-Acetylcysteine or N, N diacetylcystine. The parent compound and metabolites may be present in the plasma either free or protein bound. Renal clearance accounts for about 30 % of total body clearance. Acetylcysteine is rapidly absorbed from the gastrointestinal tract and peak plasma concentrations occur about 0.5 to 1 hour after oral administration of doses of 200 to 600 mg. Oral bioavailability is low and mean values have ranged from 4 to 10 % depending on whether total acetylcysteine or just the reduced forms are measured. It has been suggested that acetylcysteines low oral bioavailability may be due to metabolism in the gut wall and first-pass metabolism in the liver.
INDICATIONS :
1. MUCOTYLE is indicated as an adjuvant treatment in certain clinical conditions characterized by the presence of thick and viscous mucoid or mucopufulent secretions such as :
Chronic bronchopulmonary diseases (chronic obstructive pulmonary disease, emphysema with bronchiectasis).
Acute bronchopulmonary diseases (asthma with bronchial mucous plugging, bronchitis, bronchopneumonia, tracheobronchitis, bronchitis, pulmonary complications of cystic fibrosis, pulmonary complications associated with surgery).
2. To prevent Radiographic-Contrast-Agent-Induced Reductions in renal function.
3. (i) Antidote to paracetamol poisoning.
(ii) Ratol poisoning (Yellow Phosphorus Poisoning).
(iii) Carbon tetrachloride poisoning.
4. Additional indications : As adjuvant to NSAID therapy, chemo-drugs, radiation therapy, alcohol induced damage to liver, cirrhosis of liver, antibiotic therapy, biliary obstruction, jaundice etc.
Administration :
MUCOTYLE is administered orally preferably after meals, or as directed by the physician.
Dosage :
As a mucolytic agent :
Adults :
600 mg two times a day. Children : Half a tablet two times a day. The duration of treatment should be 5 to 10 days in the acute treatment, whereas it may be continued in the chronic states for several months intermittantly, according to the advice of the physician. To prevent Radiographic-Contrast-Agent-Induced
Reductions in renal function :
600 mg two times a day to be taken one day prior to procedure and on the day of procedure.
Antidote to paracetamol poisoning :
Initially 140 mg/kg, followed by 70 mg/kg every 4 hours for an additional 17 doses. As an antidote, N-acetylcysteine is reported to be very effective when administered within 8 hours of paracetamol overdose, with the protective effect diminishing after this time. Initiation of treatment after a lapse of 15 hours has previously thought to be ineffective, but recent studies suggest that beneficial results may still be obtained.
CONTRAINDICATIONS :
MUCOTYLE is contraindicated in patients with hypersensitivity or previous anaphylactic reaction to acetylcysteine or any component of the preparation. There are no contraindications to oral or I.V. administration of acetycysteine in the treatment of acetaminophen overdose.
WARNINGS :
After proper administration of acetylcysteine, an increased volume of liquified bronchial secretions may occur. When cough is inadequate, the airway must be maintained open by mechanical suction if necessary. When there is a mechanical block due to foreign body or local accumulation, the airway should be cleared by endotracheal aspiration, with or without bronchoscopy. Asthmatics under treatment with acetylcysteine should be watched carefully. Most patients with bronchospasm are quickly relieved by the use of a bronchodilator given by nebulization. If bronchospasm progresses, the medication should be discontinued immediately. Generalized urticaria has been observed rarely in patients receiving oral acetylcysteine for acetaminophen overdose. If this occurs and other allergic symptoms
appear, treatment with acetylcysteine should be discontinued unless it is deemed essential and the allergic symptoms cannot be otherwise controlled. If encephalopathy due to hepatic failure is evident, acetylcysteine treatment should be discontinued to avoid further administration of nitrogenous substances. There are no data indicating acetylcysteine adversely influences hepatic failure; however, this remains a theoretical possibility.
PRECAUTIONS :
The possible presence of a sulphurous odour does not indicate an alteration of the product but is a characteristic of the active ingredient contained in this preparation. Occasionally severe and persistent vomiting occurs as a symptom of acute acetaminophen overdose.
Treatment with oral acetylcysteine may aggravate the vomiting. Patients at risk of gastric haemorrhage (e.g., oesophageal varices, peptic ulcers, etc.) should be evaluated concerning the risk of upper gastrointestinal haemorrhage versus the risk of developing hepatic toxicity and treatment with acetylcysteine given accordingly. Dilution of the acetylcysteine with cola drinks minimizes the propensity of oral acetylcysteine to aggravate vomiting.
SPECIAL PRECAUTIONS :
MUCOTYLE should be used with caution in asthma or where there is a history of bronchospasm. It should also be used with caution with patients with a past history of oesophageal varices and peptic ulceration (acetylcysteine-induced vomiting may increase the risk of haemorrhage).
Pregnancy : Category B
Reproduction studies of N-Acetylcysteine with isoproternol have been performed in rats and of N-Acetylcysteine alone in rabbits at doses upto 2.6 times the human dose. These have revealed no evidence of impaired fertility or harm to the foetus due to N-Acetylcysteine. There are however no adequate and well-controlled studies in pregnant women. Because animal reproduction studies may not always be predictive of human responses, this drug should be used during pregnancy only if clearly needed.
Nursing Mothers :
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when acetylcysteine is administered to a nursing woman.
Drug/Laboratory Interaction :
Acetylcysteine may cause a false-positive reaction with reagent dipstick tests for urinary ketones.
Elderly Use :
There are no adequate or well controlled studies in elderly patients. For this reason, the safety and effectiveness of MUCOTYLE in the elderly has not been established.
Renal/Hepatic Impaired Patients :
Caution should be taken when administering acetylcysteine in patients with hepatic or renal failure, since there is little data relating to the effects of acetylcysteine in impaired renal and/or hepatic function. The decision to administer should be passed on a risk/benefit assessment for the individual subject. In the presence of hepatic failure due to paracetamol overdose the degree of existing liver damage and the possible risk associated with the administration of acetylcysteine should be considered.
SIDE EFFECTS :
Adverse effects have included stomatitis, nausea, vomiting, fever, rhinorrhea, drowsiness, clamminess, chest tightness and bronchoconstriction. Clinically overt acetylcysteine induced bronchospasm occurs infrequently and unpredictably even in patients with asthmatic bronchitis or bronchitis complicating bronchial asthma. Oral or I.V. administration of acetylcysteine, especially in the large doses needed to treat acetaminophen overdose, in order of frequency may result in nausea, vomiting and other gastrointestinal symptoms.
Bronchospasm may occur in conjunction with a generalised anaphylactoid reaction. The symptoms of the anaphylactic-like reaction to acetylcysteine include airway obstruction (bronchospasm), angioedema, dyspnoea, hypotension, shock, tachycardia, urticaria, and injection site reaction (including rash). These reactions occur most commonly either during, or at the end of the period of the loading dose infusion and may in fact be dose-related. Since these anaphylactic-like reactions usually occur following the loading dose, careful monitoring is recommended.
OVERDOSAGES :
Symptoms following overdosage with acetylcysteine have been similar to those of anaphylactoid reactions noted under Side Effects, but they may be more severe. Hypotension appears to be especially prominent. There is also a theoretical risk of hepatic encephalopathy.
TREATMENT OF OVERDOSAGES :
There is no specific treatment. General supportive measures should be carried out. It has been suggested that generalised reactions to acetylcysteine can be treated with intravenous injection of an antihistamine and infusion of acetylcysteine should be temporarily stopped but can be restarted at a slower rate without further reaction.
STORAGE :
Store below 30oC (86oF), protected from moisture and light.
Do not refrigerate.
SHELF LIFE :
36 months from the date of manufacture.
PRESENTATION :
MUCOTYLE tablet contains Acetylcysteine USP 600 mg.
2 Blisters of 12 tablets in a carton.
(With perforation of 4 tablets for easy dispensing).
Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.

 Cardiovascular
Cardiovascular