100 IU/ml
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
KALTO is a synthetic polypeptide of 32 amino acids in same linear sequence that is found in calcitonin of salmon origin. It is obtained by chemical synthesis and is available as an acetate. The molecular formula is C145H240N44O48S2 and molecular weight is 3432.
STRUCTURAL FORMULA :
Its structural formula is :
-Structure.jpg)
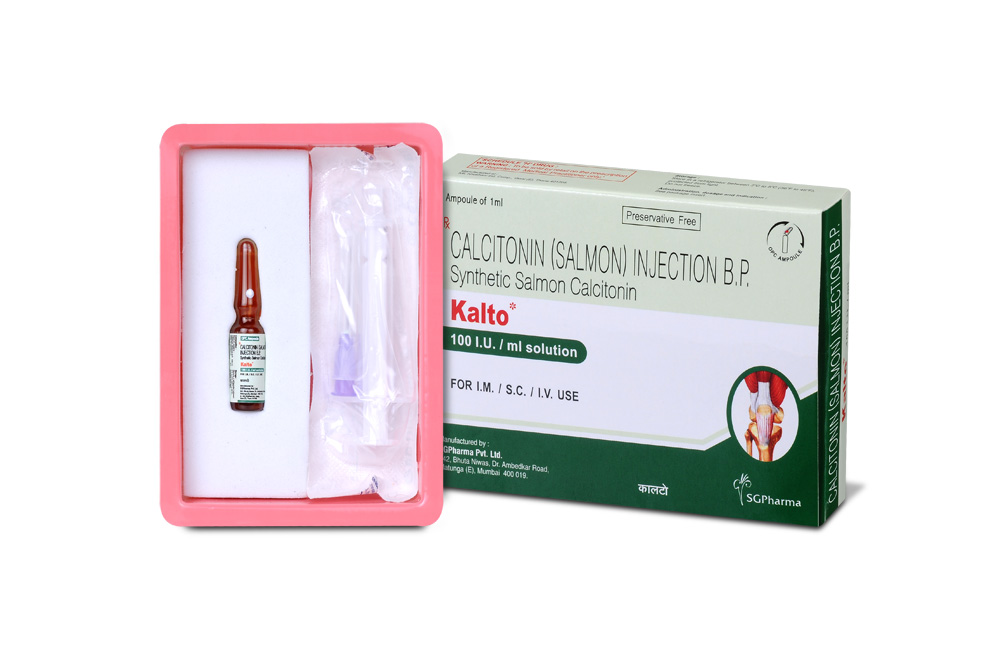
KALTO is a clear colourless solution filled in 1 mL amber glass ampoule.
COMPOSITION :
Each mL contains :
Calcitonin (Salmon) B.P. 100 I.U.
Water for Injection I.P. q.s.
ACTIONS :
Calcitonin (salmon) is a major regulating factor in mineral and skeletal metabolism; it interferes with the action of parathormone in the maintenance of the skeletal mass, by acting both on the bone and on calcium homeostasis. It markedly reduces the removal of calcium from bone in conditions with a greatly increased rate of bone resorption and formation, such as Paget`s disease, malignant osteolysis and some forms of osteoporosis characterized by a high bone turnover. Osteoclast activity inhibits osteolysis, thus lowering the abnormally increased serum calcium. Additionally, it increases the urinary excretion of calcium, phosphorus and sodium by reducing their tubular reuptake. Serum calcium, however, is not reduced below the normal range. Calcitonin (salmon) reduces gastric and exocrine pancreatic secretion without influencing gastrointestinal motility. Clinical experience demonstrates that calcitonin (salmon) possesses analgesic activity. Investigations have shown specific calcitonin (salmon) binding sites in some areas of the central nervous system. All calcitonin structures show 32 amino-acids in a single chain, the sequence of which differs from species to species. Due to its greater affinity to receptor binding sites than synthetic calcitonins from mammalian species, including the synthetic human calcitonin, calcitonin is more potent and longer acting clinically.
PHARMACOKINETICS :
Absorption :
Calcitonin (salmon) is rapidly absorbed and eliminated.
Distribution :
The apparent volume of distribution is 0.15-0.3 L/kg and the protein binding is 30-40 %. Maximal plasma concentrations are obtained within one hour of administration. Bioavailability following subcutaneous and intramuscular injection in humans is high and similar for the two routes of administration (71 % and 66 %, respectively). Have short absorption and elimination half-lives of 10-15 minutes and 50-80 minutes.
Metabolism :
Calcitonin (salmon) and its metabolites are excreted up to 95 % by the kidney, the percentage of parent drug being 2 %. Animal studies have shown that calcitonin is primarily metabolized via proteolysis in the kidney following parenteral administration. The metabolites lack the specific biological activity of calcitonin.
Excretion :
Calcitonin (salmon) is primarily and almost exclusively degraded in the kidneys, forming pharmacologically inactive fragments of the molecule. Therefore, the metabolic clearance is much lower in patients with end-stage renal failure than healthy subjects. However, the clinical relevance of this finding is not known.
Characteristics in patients :
There is a relationship between the subcutaneous dose of calcitonin (salmon) and the peak plasma concentrations. Following parenteral administration of 100 I.U. calcitonin (salmon), peak plasma concentration lies between about 200 and 400 pg/mL. Higher blood
levels are associated with increased incidence of nausea and vomiting.
INDICATIONS :
• Hypercalcaemia and hypercalcaemic crisis due to tumoral osteolysis secondary to breast, lung, kidney and other malignancies, due to osteolysis induced by myeloma, due to hyperparathyroidism, immobilization and vitamin D intoxication, both for treatment of emergencies and for prolonged treatment.
• Paget`s disease of bone (osteoitis deformas), particularly in cases with bone pain, with neurological complications, with increased bone turnover reflected in elevated alkaline phosphatases and hydroxyproline excretion, with progressive extension of bone lesions, with
incomplete or repeated fractures.
• Osteoporosis with high bone turnover due to oestrogen deficiency, as well as primary or secondary hyperparathyroidism.
• Bone pain associated with osteoporosis, particularly acute and chronic bone pain due to vertebral and other fractures and with osteolysis due to malignancies.
• Neurodystrophic disorders (synonymous algodystrophy or Sudeck`s disease), due to various aetiological and predisposing factors such as post-traumatic painful osteoporosis, reflex dystrophy, shoulder-arm syndrome, causalgia, druginduced neurotrophic disorders.
Administration :
Calcitonin (salmon) may be administered subcutaneously, intramuscularly or intravenously; local and systemic tolerance is good with all 3 routes of administration and in the recommended dosage. If the volume of calcitonin (salmon) injection to be injected exceeds 2 mL, intramuscular injection is preferable and multiple sites of injection should be used. Calcitonin (salmon) may be administered at bedtime to reduce the incidence of nausea or vomiting which may occur, especially at the initiation of therapy. Allow the contents of the ampoule to reach room temperature before intramuscular or subcutaneous use.
INSTRUCTIONS FOR USE OF AMPOULE :
The ampoule used in this product is equipped with O.P.C (One Point Cut) opening system. No ampoule file is needed to open the ampoule. The neck of the ampoule is prescored at the point of constriction. A coloured dot on the ampoule head helps to orientate the ampoule. Take the ampoule and face the coloured dot. Let the solution at the head of the ampoule to flow down by shaking or a gentle stroke. The ampoule opens easily by placing the thumb on the coloured dot and gently pressing downwards as shown.

Dosage :
Hypercalcaemia :
• Emergency treatment of hypercalcaemic crisis Intravenous drip infusion is the most effective method of administration and should always be used in emergencies or severe cases. 5-10 I.U. calcitonin per kg body weight daily and administered by slow I.V. infusion in 500 mL normal saline over at least six hours, or by slow intravenous injection in 2 to 4 divided doses spread over the day. Rehydration should be considered. Emergency treatment is followed by specific treatment of the underlying disease, if required. • Prolonged treatment in chronic hypercalcaemic states. Treatment should be adjusted to the patients clinical and biochemical response. Usually 5-10 I.U. per kg by subcutaneous or intramuscular injection in one or two single doses.
Paget`s disease :
100 I.U. daily by subcutaneous or intramuscular injection. In some cases the injections may be given only every second day. In particular after improvement of the objective and subjective symptoms, an injection of 50 I.U. per day may be sufficient.
Osteoporosis with high bone turnover :
50-100 I.U. daily or every second day administered as a subcutaneous or intramuscular injection.
Bone pain associated with osteoporosis and osteolysis :
The dosage should be adjusted to the individual needs.
• Bone pain associated with osteolysis 100-200 I.U. daily, which may be given as I.V. infusion or as one or two single doses by subcutaneous or intramuscular injections. The initial dosage should be maintained until a satisfactory response is noted. The maintenance is determined either by decreasing the initial dosage of calcitonin (salmon) or by increasing the time interval between administrations.
• Bone pain associated with osteoporosis 50-100 I.U. daily or every second day administered as a subcutaneous or intramuscular injection.
Neurodystrophic disorders :
100 I.U. by the subcutaneous or intramuscular route daily for 2-4 weeks, followed by 3 injections of 100 I.U. per week for a treatment period of up to 6 weeks depending on clinical progress. Early diagnosis is essential and treatment should start once the diagnosis is confirmed.
NOTES :
In Paget`s disease and other chronic conditions with high bone turnover, treatment should be given for periods ranging from several months up to a few years. Treatment markedly reduces blood alkaline phosphatases and urinary hydroxyproline excretion levels may rise after an initial fall; the physician must then judge from the clinical picture whether treatment should be continued. Disorders of bone metabolism may recur one or several months after treatment has been discontinued, necessitating a new course of calcitonin (salmon).
Patients who are instructed in the self administration of subcutaneous injections must receive precise directions from the physician or the nurse. Antibodies to calcitonin (salmon) may develop in a few patients under long-term therapy. Clinical efficacy, however, is usually not affected. Escape phenomena seen sometimes in long-term therapy are probably due to a saturation of the binding sites and not apparently related to the development of antibodies. After an interruption of treatment the therapeutic response to calcitonin (salmon) is restored.
CONTRAINDICATIONS :
Hypersensitivity to synthetic calcitonin (salmon) or to any of the excipients of the formulation.
WARNINGS :
Because calcitonin (salmon) is protein in nature, the possibility of a system allergic reaction exists. Administration of calcitonin (salmon) has been reported in a few cases to cause serious allergictype reactions (e.g. bronchospasm, swelling of the tongue or throat, and anaphylactic shock), and in one case, death attributed to anaphylaxis. The usual provisions should be made for the emergency treatment of such a reaction should it occur. Allergic reactions should be differentiated from generalized flushing and hypotension. For patients with suspected sensitivity to calcitonin (salmon), skin testing should be considered prior to treatment utilizing a dilute, sterile solution of calcitonin (salmon). Physicians may wish to refer patients who require skin testing to an allergist.
PRECAUTIONS :
The administration of calcitonin (salmon) possibly could lead to hypocalcaemic tetany under special circumstances although no cases have yet been reported. Provision for parenteral calcium administration should be available during the first several administrations of calcitonin (salmon). Prolonged treatment of bedridden patients must be accompanied by regular (at least monthly) checks of urinary sediment. Nausea, vomiting, dizziness, slight facial flushing accompanied by a sensation of heat and arthralgia has been reported. Nausea, vomiting, flushing and dizziness are dose dependent and are more frequent after I.V. than after I.M. or S.C. administration. There have been rare reports of polyuria and chills. These effects usually subside spontaneously and a temporary dose reduction is necessary in few cases only. In rare cases, calcitonin (salmon) may give rise to hyper sensitivity reactions; these include local effects at the injection site or generalized skin reactions. Allergic reactions manifested in some cases by rash, hypertension or peripheral oedema. Anaphylactoid-like reactions and single cases of anaphylactic shock have been reported. Side effects occur less frequently with intranasal than with parenteral administration of calcitonin (salmon).
During the first few days of the drug administration, some patients may experience diuresis and increased urinary sodium excretion but this usually returns to the baseline levels within 5 to 7 days. Urinary frequency may occur during this time. Antibodies to calcitonin (salmon) have been reported in 30 to 50 % of patients after 2 to 18 months of therapy, but in no instance have these elevated antibody titers been associated with any systemic allergic or anaphylactic effect, and only rarely have antibodies been associated with the
development of clinical resistance to calcitonin (salmon).
Pregnancy : Category C
Calcitonin (salmon) has been shown to cause a decrease in foetal birth weights in rabbits when given in doses 14 - 56 times the dose recommended for human use. Since calcitonin (salmon) does not cross the placental barrier, this finding may be due to metabolic
effects on the pregnant animal. There are no adequate and well-controlled studies in pregnant women. KALTO should be used during pregnancy only if the potential benefits justifies the potential risk to the foetus.
Nursing mothers :
Calcitonin (salmon) inhibits lactation in animals, and the drug should therefore not be administered to nursing mothers.
Paediatric Use :
Disorders of bone in children referred to as juvenile Pagets disease have been reported rarely. The relationship of these disorders to adult Pagets diseases has not been established and experience with the use of calcitonin (salmon) in these disorders is very limited. There is no adequate data to support the use of KALTO in children.
INTERACTIONS :
Serum calcium levels may be transiently decreased to below normal levels following administration of calcitonin (salmon), notably upon initiation of therapy
SIDE EFFECTS :
Frequency categories : Very common ( > 1/10); common ( > 1/100, < 1/10); uncommon ( > 1/1,000, < 1/100); rare ( > 1/10,000; < 1/1,000); very rare(< 1/10,000).
Gastrointestinal disorders : Very common : Nausea with or without vomiting is noted in approximately 10 % of patients treated with calcitonin (salmon). The effect is more evident on initiation of therapy and tends to decrease or disappear with continued administration or a reduction in dose. An antiemetic may be administered, if required. Nausea, vomitting are less frequent when the injection is done in the evening and after meals.
Vascular disorders : Very common : skin flushes ( facial or upper body). These are not allergic reactions but are due to pharmacological effect, and are usually observed 10 to 20 minutes after administration. General disorders and administration site
Uncommon : Local inflammatory reactions at the site of subcutaneous or intramuscular injection.
Metabolic and nutrition disorders : Rare : In case of patients with high remodelling (Pagets disease and young patients) a transient decrease of calcemia may occur between the 4th and the 6th hour after administration, usually asymptomatic.
Immune system disorders : Very rare : Serious allergic - type reactions, such as bronchospasm, swelling of the tongue and throat, and in isolated cases, anaphylaxis.
INFORMATION FOR PATIENTS :
Patient should not drive or use machine because calcitonin (salmon) may cause transient dizziness.
OVERDOSAGE :
Nausea, vomiting, flushing and dizziness are known to be dose dependent when calcitonin (salmon) is administered parenterally. Single dose (up to 10,000 I.U.) of injectable calcitonin (salmon) have been administered without adverse reactions, other than nausea and vomiting, and exacerbation of pharmacological effects. No serious consequences due to overdosage have so far been reported.
TREATMENT OF OVERDOSAGE :
Treatment would be symptomatic.
PHARMACEUTICAL PRECAUTIONS :
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
STORAGE :
Store in a refrigerator between 2°C to 8°C (36°F to 46°F), protected from light.
Do not freeze.
SHELF LIFE :
24 months from the date of manufacture.
PRESENTATION :
KALTO is supplied as 100 I.U. calcitonin (salmon) in 1 mL amber glass ampoule. Such 1 ampoule is packed in a box.
Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.

 Cardiovascular
Cardiovascular