10 mg/2 ml, 20 mg/2 ml
40 mg/2 ml, 60 mg/2 ml
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
POLICAN (Polidocanol), also known as Laureth-9, is an emulsifying agent. When it is injected into a vessel it damages the endothelium resulting in a thrombosis. In combination with compression bandaging, polidocanol can be used to treat varicose veins in the legs. As polidocanol has some anaesthetic effects this scelerotherapy is relatively painless. Chemically polidocanol is macrogol lauryl ether. Its molecular formula is C30H62O10 and its molecular weight is 582.00.
STRUCTURAL FORMULA :
Its structural formula is :
C12H25O(CH2CH2O) ~9H
A clear, colourless to faintly yellowish-green sterile aqueous solution filled in ampoule of suitable size.
COMPOSITION :
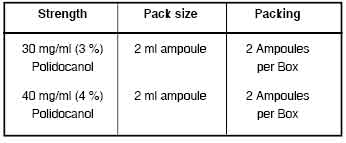
For 3 % Injection
Each ml contains :
Polidocanol 30 mg
Absolute Alcohol I.P. 5 % v/v
Water for Injections I.P. q.s.
For 4 % Injection
Each ml contains :
Polidocanol 40 mg
Absolute Alcohol I.P. 5 % v/v
Water for Injections I.P. q.s.
ACTIONS :
Polidocanol has a sclerosant and at the same time a local anaesthetic effect. This permits almost painless sclerotherapy. The effect is directed mostly at the vein intima. While there is great affinity with damaged intima there is no effect on intact vascular linings, so a sclerosant effect occurs only on veins with actual varicose changes and not on veins with moderate cylindrical dilation. This sclerosant effect is due to irritation of the vein intima, which causes a local, adhesive thrombosis in the area of the damaged endothelium. The compression bandage applied after treatment presses the vein walls together and prevents re-canalisation of the organised thrombus, so that the desired conversion of the thrombus into a fibrous scar takes place. With paravasal application of Polidocanol, the local oedema formation leads, to compression of the varices and cicatricial consolidation. With appropriate selection of concentration and dosage and with correct treatment methods and aftercare (compression treatment) POLICAN can be easily tolerated and is a reliable and lasting sclerosant.
PHARMACOKINETICS :
12 hours after intravenous application, about 90 % of the Polidocanol administered will have been eliminated from the blood. No accumulation is to be expected even after repeated doses of Polidocanol at intervals which are normal for sclerotherapy. In a study, the following values were determined after a single intravenous dose : protein binding 64 %, terminal elimination half-life 4 hours, volume of distribution 24.5 l, total clearance 11.7 l/h, renal clearance 2.43 l/h and biliary clearance 3.14 l/h.
INDICATIONS :
POLICAN 3 % Sclerotherapy of medium-sized to large varices. Sclerotherapy of haemorrhoidal disease (1st and 2nd degree).
POLICAN 4 % Sclerotherapy of medium-sized to large varices (collateral varices and insufficiency of the saphenous veins; as a complementary treatment to surgical intervention or if surgical treatment is not indicated). Sclerotherapy of haemorrhoidal disease (1st and 2nd degree).
Administration :
For Intravenous Injection (varices), and for submucous Injection (haemorrhoidal disease). Irrespective of the mode of venepuncture (in a standing patient with the cannula only or in a sitting patient with a syringe ready for injection), injections should only be carried out in a leg placed horizontally or elevated approx. 30-45° above the horizontal. Injections must be strictly intravenous. Depending on the degree and extent of the varices, several treatments may be required at intervals of 1-2 weeks. Note : Thrombi, which occasionally develop, are removed by stab incision and thrombus expression.
INSTRUCTION FOR USE OF AMPOULE :
The ampoule used in this product is equipped with O.P.C. (One Point Cut) opening system. No ampoule file is needed to open the ampoule. The neck of the ampoule is prescored at the point of constriction. A coloured dot on the ampoule head helps to orientate the ampoule. Take the ampoule and face the coloured dot. Let the solution at the head of the ampoule to flow down by shaking or a gentle stroke. The ampoule opens easily by placing the thumb on the coloured dot and gently pressing downwards as shown.

Dosage :
Due to the lack of sufficient data, a positive benefit-risk assessment and dosage recommendation cannot yet be given for the use of POLICAN in foam sclerotherapy. Dosage of single and daily doses. Generally, the dose of 2 mg macrogol lauryl ether (polidocanol) per kg body weight per day should not be exceeded. For a patient weighing 70 kg, a total of up to 140 mg macrogol lauryl ether (polidocanol) can be injected. 140 mg macrogol lauryl ether (polidocanol) are contained in :
POLICAN 3 % 4.6 ml.
POLICAN 4 % 3.5 ml.
The dosages actually administered are usually well below the maximum quantities specified. Extensive varicosis should always be treated in several sessions. When treating a patient with predisposition to hypersensitivity reactions for the first time, no more than one injection should be administered. Depending on the response, several injections may be administered in subsequent treatment sessions, provided that the maximum dose is not exceeded.
POLICAN 3 %
Sclerotherapy of medium-sized varices :
Depending on the diameter of the varices to be treated, POLICAN 2 % or 3 % is used. In the first treatment, only one injection of 0.5-1 ml POLICAN 2 % or 3 % should be administered. Depending on the outcome and the length of the segment to be treated, several injections with up to 2 ml per injection may be administered in subsequent treatment sessions, provided that the maximum dose is not exceeded. POLICAN 3 % and 4 %.
Sclerotherapy of large varices :
In the first treatment, only one injection of 1 ml POLICAN 3 % or 4 % is administered. Depending on the outcome and the length of the segment to be treated, several injections (2-3) with upto 2 ml per injection may be administered in subsequent treatment sessions, provided that the maximum dose is not exceeded.
Sclerotherapy of haemorrhoidal disease :
During one treatment session, a total of 3 ml POLICAN 3 % or 2.5 ml POLICAN 4 % should not be exceeded. Depending on the findings, a maximum of 1 ml per haemorrhoid is administered as a strictly submucous injection. When treating an 11 o’clock haemorrhoid in men, the quantity injected must not exceed 0.5 ml. In the case of external haemorrhages the injection must be placed subcutaneously (application drop by drop and not into the knot itself).
COMPRESSION TREATMENT AFTER INJECTION OF POLICAN :
Once the injection site has been covered, a tight compression bandage or elastic stocking must be applied. After that, the patient should walk for 30 minutes, preferably within reach of the practice. For extensive varicosis, compression treatment with short traction bandages for several months is recommended.After use of POLICAN 3 % or 4 %, compression should be applied for 3-5 weeks.To make sure the bandage does not slip, especially on the thigh and conical limbs, a foam bandage support under the actual compression bandage is recommended.The success of sclerotherapy relies on thorough and careful follow-up compression treatment.
WARNINGS :
All POLICAN products contain 5 % (v/v) alcohol. This must be taken into account in patients with previous alcoholism. POLICAN products contain potassium, but less than 1 mmol (39 mg) potassium per ampoule. POLICAN products contain sodium, but less than 1 mmol (23 mg) sodium per ampoule.
PRECAUTION :
SCLEROTHERAPY OF VARICES :
Sclerosants must never be injected intra-arterially because this can cause severe necroses which may necessitate amputation. A vascular surgeon must be called in immediately if any such incidents occur (see overdosages). An indication in the facial area must be strictly evaluated for all sclerosants because intravascular injection can lead to pressure reversal in the arteries and hence to irreversible visual disturbances (blindness). In certain body regions such as in the foot or malleolar region, the risk of inadvertent intra-arterial injection may be increased. Therefore, only small amounts should be used in low concentrations with particular care during treatment.
Pregnancy :
There are no adequate data from the use of POLICAN in pregnant women. Studies in animals showed reproductive toxicity, but no teratogenic potential. Therefore, POLICAN must not be used during pregnancy unless clearly necessary.
Lactation :
Investigations on the possible excretion of macrogol lauryl ether (polidocanol) in the breast milk have not been performed in humans. If sclerotherapy is necessary during breast-feeding, it is advisable to postpone breast-feeding for 2-3 days.
EFFECTS ON ABILITY TO DRIVE AND OPERATE MACHINES :
No negative effects on the ability to drive and operate machines are known for POLICAN.
INTERACTION :
Macrogol lauryl ether (polidocanol) is a local anaesthetic. When combined with other anaesthetics, there is a risk of an additive effect of these anaesthetics on the cardiovascular system.
SIDE EFFECTS :
Sclerotherapy of varices :
Local adverse reactions (e.g. necroses), especially of the skin and of the underlying tissue (and, in rare cases, of the nerves), were observed when treating varices in the leg after inadvertent injection into the surrounding tissue (paravenous injection). The risk increases with increasing POLICAN concentrations and volumes. In addition, the following adverse reactions were observed with the frequencies seen below :
Very common (= 10 %); common (= 1 %-< 10 %); uncommon (= 0.1 %-< 1 %); rare (= 0.01 %-< 0.1 %); very rare, including isolated cases (< 0.01 %).
Immune system disorders :
Very rare : anaphylactic shock, angioneurotic oedema, urticaria generalised, asthma.
Nervous system disorders :
Very rare : headache, migraine, paraesthesia (local), loss of consciousness, confusional state, dizziness
Eye disorders :
Very rare : visual disturbance.
Cardiac disorders :
Very rare : palpitations.
Vascular disorders :
Common : neovascularisation, haematoma.
Uncommon : thrombophlebitis superficial, phlebitis.
Rare : deep vein thrombosis (unknown aetiology, possibly due to the underlying disease).
Very rare : pulmonary embolism, syncopevasovagal, circulatory collapse, vasculitis.
Respiratory, thoracic and mediastinal disorders :
Very rare : dyspnoea, chest discomfort.
Gastrointestinal disorders :
Very rare : dysgeusia, nausea.
Skin and subcutaneous tissue disorders :
Common : skin hyperpigmentation, ecchymosis.
Uncommon : dermatitis allergic, urticaria contact, skin reaction, erythema.
Very rare : hypertrichosis (in the area of sclerotherapy).
General disorders and administration site conditions :
Common : injection site pain (short-term), injection site thrombosis (local intravaricose blood clots).
Uncommon : necrosis, induration, swelling.
Very rare : pyrexia, hot flush investigations.
Very rare : blood pressure abnormal injury, poisoning and procedural complications.
Uncommon : nerve injury.
OVERDOSAGE AND TREATMENT :
Emergency measures and antidotes.
Anaphylactic reactions :
Anaphylactic reactions are rare, but potentially life-threatening situations. The attending doctor should be prepared for emergency measures and have a suitable emergency kit available. Treatment of local intoxication after improper administration when treating varices of the legs.
PHARMACEUTICAL PRECAUTION :
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
STORAGE :
Store below 30°C (86°F), protected from light.
Do not refrigerate.
SHELF LIFE :
36 months from the date of manufacture.
Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.

 Cardiovascular
Cardiovascular