5,000 IU, 10,000 IU
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
L-ASPERONE ( L-asparaginase) is derived from Escherichia coli and used as an antineoplastic agent for treatment of leukaemia. L-ASPERONE is a white lyophilized cake in amber tubular vial of suitable size.
COMPOSITION :
L-ASPERONE
Each vial contains :
L-asparaginase 5,000 IU
(Lyophilized)
L-ASPERONE
Each vial contains :
L-asparaginase 10,000 IU
(Lyophilized)
ACTIONS :
L-asparaginase catalyzes the hydrolysis of asparagine in serum to aspartic acid and ammonia. Asparagine is an amino acid necessary for the synthesis of protein and the multiplication and growth of cells. Normal cells are able to synthesize asparagine themselves, however tumor cells, more specifically lymphatic tumor cells have no such ability. With the rapid decrease of asparagine, and also because tumor cells can neither get enough asparagine from blood, nor make it themselves, the production of protein and the multiplication of cells are inhibited, the tumor cells destruct and die. L-asparaginase also interferes with the synthesis of DNA and RNA and affects the multiplication phase Gl of cells, and acts to inhibit cell segmentation.
PHARMACOKINETICS :
L-asparaginase is absorbed when administered by either intravenous or intramuscular route. It is 30 % bound with plasma proteins. It is detected in lymph after absorption, but has a low concentration in cerebrospinal fluid. After dosing, the concentration of asparagine in blood drops rapidly to a level that cannot be detected, indicating its quick absorption in the body. Given by intramuscular and intravenous injection, plasma half-life (t1/2) are 39 to 49 hours and 8 to 30 hours respectively. Peak plasma levels of L-asparaginase is reached 14 to 24 hours after intramuscular injection. Asparagine can still be detected in plasma after treatment has been stopped for 23 to 33 days. Its excretion seems double positive and only trace amount appear in the urine.
INDICATIONS :
L-asparaginase is indicated in the therapy of Acute Lymphoblastic Leukaemia (ALL), acute granulocytic leukaemia, Hodgkin’s disease and non - Hodgkin’s disease lymphoma, melanoma. It acts by inhibiting the multiplication of the above-mentioned tumor cells. It has a good curative effect on children with ALL in their induction and remission period, sometimes also used in patients apt to recrudesce after relieving by chemotherapeutic medication. It has comparatively shorter remission period and easily induces drug resistance when used alone, and hence it is used in combination with other chemotherapeutic agents to improve therapeutic effect.
Administration :
For intravenous and intramuscular use.
Dosage :
This drug may have toxic properties and must be handled and administered with care. Special handling procedures should be reviewed prior to handling and followed diligently during reconstitution and administration. Inhalation of dust or aerosols and contact with skin or mucous membranes, especially those of the eyes, must be avoided. As a component of selected multiple agent induction regimens, L-ASPERONE may be administered by either the intravenous or the intramuscular route. When administered intravenously this enzyme should be given over a period of not less than thirty minutes through the side arm of an already running infusion of Sodium Chloride Injection or Dextrose Injection 5 % (D5W), L-aSPERONE has little tendency to cause phlebitis when given intravenously. Anaphylactic reactions require the immediate use of epinephrine, oxygen and intravenous steroids. When administering L-ASPERONE intramuscularly, the volume at a single injection site should be limited to 2 ml. If a volume greater than 2 ml is to be administered, two injection sites should be used.
Unfavorable interactions of L-ASPERONE with some antitumor agents have been demonstrated. It is recommended therefore, that L-ASPERONE be used in combination regimens only by physicians familiar with the benefits and risks of a given regimen. During the period of its inhibition of protein synthesis and cell replication, L-ASPERONE may interfere with the action of durgs such as methotrexate which require cell replication for their lethal effect. L-ASPERONE may interfere with the enzymatic detoxification of other drugs, particularly in the liver.
Recommended Induction Regimens :
When using chemotherapeutic agents in combination for the induction of remissions in patients with acute lymphocytic leukaemia, regimens are sought which provide maximum chance of success while avoiding excessive cumulative toxicity or negative drug interactions.
One of the following combination regimens incorporating L-ASPERONE is recommended for acute lymphocytic leukaemia in paediatric patients : In the regimens below, Day 1 is considered to be the first day of therapy.
Regimen I
Prednisone 40 mg/square meter of body surface area per day orally in three divided doses for 15 days, followed by tapering of the dosage as follows : 20mg/square meter for 2 days, 10 mg/square meter for 2 days, 5 mg/square meter for 2 days, 2.5 mg/square meter for 2 days and then discontinue. Vincristine sulfate 2 mg/square meter of body surface area intravenously once weekly on Days 1, 8 and 15 of the treatment period. The maximum single dose should not exceed 2.0 mg. L-asparaginase 1,000 I.U./kg/day intravenously for ten successive days beginning on Day 22 of the treatment period.
Regimen II
Prednisone 40 mg/square meter of body surface area per day orally in three divided doses for 28 days (the total daily dose should be to the nearest 2.5 mg), following which the dosage of prednisone should be discontinued gradually over a 14 day period. Vincristine sulfate 1.5 mg/square meter of body surface area intravenously weekly for four doses, on Days 1,8,15, and 22 of the treatment period. The maximum single dose should not exceed 2.0 mg. L-asparaginase 6,000 I.U./square meter of body surface area intramuscularly on Days 4,7,10,13,16,19,22,25, and 28 of the treatment period. When a remission is obtained with either of the above regimens, appropriate maintenance therapy must be instituted. L-ASPERONE should not be used as part of a maintenance regimen. The above regimens do not preclude a need for special therapy directed toward the prevention of central nervous system leukaemia. It should be noted that L-ASPERONE has been used in combination regimens other than those recommended above. It is important to keep in mind that L-ASPERONE administered intravenously concurrently with or immediately before a course of vincristine and prednisone may be associated with increased toxicity. Physicians using a given regimen should be thoroughly familiar with its benefits and risks. Clinical data are insufficient for a recommendation concerning the use of combination regimens in adults. L-asparaginase toxicity is reported to be greater in adults than in paediatric patients. Use of L-ASPERONE as the sole induction agent should be undertaken only in an unusual situation when a combined regimen is inappropriate because of toxicity or other specific patient-related factors, or in cases refractory to other therapy. When L-ASPERONE is to be used as the sole induction agent for paediatric patients or adults, the recommended dosage regimen is 200 I.U./kg/day intravenously for 28 days. When complete remissions were obtained with this regimen, they were of short duration, 1 to 3 months. L-ASPERONE has been used as the sole induction agent in other regimens. Physicians using a given regimen should be thoroughly familiar with its benefits and risks.
Patients undergoing induction therapy must be carefully monitored and the therapeutic regimen adjusted according to response and toxicity. Such adjustments should always involve decreasing dosages of one or more agents or discontinuation depending on the degree of toxicity. Patients who have received a course of L-ASPERONE, if retreated, have an increased risk of hypersensitivity reaction. Therefore, retreatment should be undertaken only when the benefit of such therapy is weighted against the increased risk.
Intradermal Skin Test :
Because of the occurrence of allergic reactions, an intradermal skin test should be performed prior to the initial administration of L-ASPERONE and when L-ASPERONE is given after an interval of a week or more has elapsed between doses. The skin test solution may be prepared as follows; Reconstitute the contents of a 10,000 I.U. vial with 5.0 ml of diluent. From this solution (2,000 I.U./ml) withdraw 0.1 ml and inject it into another vial containing 9.9. ml of diluent, yielding a skin test solution of approximately 20.0 I.U./ml. Use 0.1 ml of this solution (about 2.0 I.U.) for the intradermal skin test. The skin test site should be observed for at least one hour for the appearance of a wheal or erythema either of which indicates a positive reaction. An allergic reaction even to the skin test dose in certain sensitized individuals may rarely occur. A negative skin test reaction does not preclude the possibility of the development of an allergic reaction.
Desensitization :
Desensitization should be performed before administering the first dose of L-ASPERONE on initiation of therapy in positive reactors, and on retreatment of any patient in whom such therapy is deemed necessary after carefully weighing the increased risk of hypersensitivity reactions. Rapid desensitization of the patient may be attempted with progressively increasing amounts of intravenously administered L-ASPERONE provided adequate precautions are taken to treat an acute allergic reaction should it occur. One reported schedule begins with a total of 1 I.U. given intravenously and doubles the dose every 10 minutes, provided no reaction has occurred, until the accumulated total amount given equals the planned doses for that day. For convenience the following table is included to calculate the number of doses necessary to reach the patient’s total dose for that day :
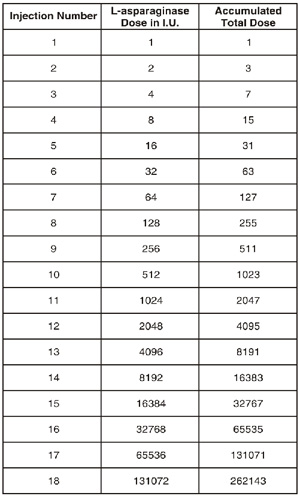
For example : A patient weighing 20 kg who is to receive 200 I.U./kg (total dose 4000 I.U.) would receive injections 1 through 12 during desensitization.
Directions for Reconstitution
This drug may have toxic properties and must be handled and administered with care. Inhalation of dust or aerosols and contact with skin or mucous membranes, especially those of the eyes, must be avoided. Appropriate protective equipment should be worn when handling L-ASPERONE.
For Intravenous Use
Reconstitute with Sterile Water for Injection or with Sodium Chloride Injection. The volume recommended for reconstitution is 5 ml for the 10,000 unit vials. Ordinary shaking during reconstitution does not inactivate the enzyme. This solution may be used for direct
intravenous administration within an eight hour period following restoration. For administration by infusion, solutions should be diluted with the isotonic solutions, Sodium Chloride Injection or Dextrose injection 5%. These solutions should be infused within eight hours and only if clear. Occasionally a very small number of gelatinous fiber-like particles may develop on standing. Filtration through a 5.0 micron filter during administration will remove the particles with no resultant loss in potency. Some loss of potency has been observed with the use of a 0.2 micron filter.
For Intramuscular Use
When L-ASPERONE is administered intramuscularly according to the schedule cited in the induction regimen, reconstitution is carried out by adding 2 ml Sodium Chloride Injection to the 10,000 unit vial. The resulting solution should be used within eight hours and only if clear.
Special Handling
L-asparaginase may be irritating to eyes, skin and the upper respiratory tract. It has been shown to be embryotoxic and teratogenic by the intravenous route in animal studies. Due to the drug’s potential toxic properties, appropriate precautions including the use of appropriate safety equipment are recommended for the preparation of L-ASPERONE for administration. Inhalation of dust or aerosols and contact with skin or mucous membranes, especially those of the eyes, must be avoided. The National Institutes of Health presently recommends that the preparation of injectable antineoplastic drugs should be performed in a Class II laminar flow biological safety cabinet. Personnel preparing drugs of this class should wear chemical resistant, impervious gloves, safety goggles, outer garments and shoe covers. Additional body garments should be used based upon the task being performed (e.g. sleevelets, apron, gauntlets, disposable suits) to avoid exposed skin surfaces and inhalation of vapors and dust. Appropriate techniques should be used to remove potentially contaminated clothing. Several other guidelines for proper handling and disposal of antineoplastic drugs have been published and should be considered.
Accidental Contact Measures
Should accidental eye contact occur, copious irrigation for at least 15 minutes with water, normal saline or a balanced salt ophthalmic irrigating solution should be instituted immediately, followed by prompt ophthalmologic consultation. Should accidental skin contact
occur, the affected part should be washed immediately with soap and water. Medical attention should be sought. If inhaled, remove from exposure and seek medical attention.
CONTRAINDICATIONS :
L-asparaginase is contraindicated in patients with :
• Previous anaphylactic reactions to it or who show positive reaction in the skin test.
• Pancreatitits or a history of pancreatitis.
• Serious varicella or zoster.
• Yellow fever vaccine.
WARNINGS :
Allergic reactions to L-asparaginase are frequent and may occur during the primary course of therapy. They are not completely predictable on the basis of the intradermal skin test. Anaphylaxis and death have occurred even in a hospital setting with experienced
observers. Once a patient has received L-ASPERONE as part of a treatment regimen, retreatment with this agent at a later time is associated with increased risk of hypersensitivity reactions. In patients found by skin testing to be hypersensitive to L-asparaginase, and in any patient who has received a previous course of therapy with L-asparaginase, therapy with this agent should be instituted or reinstituted only after successful desensitization, and then only if in the judgement of the physician the possible benefit is greater than the increased risk. Desensitization itself may be hazardous. In view of the unpredictability of the adverse reactions to L-asparaginase, it is recommended that this product be used in a hospital setting. L-asparaginase has an adverse effect on liver function in the majority of patients. Therapy with L-asparaginase may increase pre-existing liver impairment caused by prior therapy or the underlying disease. Because of this there is a possibility that L-asparaginase may increase the toxicity of other medications. The administration of L-ASPERONE intravenously concurrently with or immediately before a course of vincristine and prednisone may be associated with increased toxicity.
PRECAUTIONS :
L-asparaginase of Escherichia coli origin and that of Erwinia carotora origin may rarely have cross-allergic reactions. L-asparaginase has been reported to interfere with the interpretation of thyroid function tests by producing a rapid and marked reduction in serum concentrations of thyroxine binding globulin within two days after the first dose. Serum concentrations of thyroxine binding globulin returned to pretreatment value within four weeks of the last dose of L-asparaginase. The concentration of blood ammonia and blood urea nitrogen may rise because of the decomposition of asparagine. Blood sugar, blood uric acid and urine uric acid may rise. In the initial three weeks of treatment, partial time of thrombokinase, thrombinogen and thrombin may be extended, platelet count may rise. The concentration of plasma fibrinogen, antithrombin, proplasmin, serum albumin may be reduced as L-asparaginase inhibits the synthesis of plasma proteins. Liver function abnormality indicates symptoms of liver toxicity and disorder. Serum calcium may decrease.
To be used cautiously in patients with diabetes; gout or a history of renal urate calculus; hepatic dysfunction or infection; and patients that have received cytotoxin treatment of radiotherapy. The following should be carefully monitored before and during course of the therapy : Plasma clotting factors, blood sugar, serum amylase, blood uric acid, liver function, renal function, marrow smear classification, serum calcium, central nervous system function. As L-asparaginase can further inhibit the immune mechanism of patients and increase the multiplication, toxicity, adverse reaction of virus during inoculation, live virus bacterin inoculation should not be received within three months of treatment. Persons who are going to take poliomyelitis bacterin orally should postpone the schedule when they are in intimate contact with the patients.
Laboratory Tests :
The fall in circulating lymphoblasts often is quite marked; normal or below normal leukocyte counts are noted frequently within the first several days after initiating therapy. This may be accompanied by a marked rise in serum uric acid. The possible development of uric acid nephropathy should be borne in mind. Appropiate preventive measures should be taken, e.g. allopurinol, increased fluid intake, alkalization of urine. As a guide to the efects of therapy the patient’s peripheral blood count and bone marrow should be monitored frequently. Frequent serum amylase determinations should be obtained to detect early evidence of pancreatitis. If pancreatitis occurs, therapy should be stopped and not reinstituted. Blood sugar should be monitored during therapy with L-asparaginase because hyperglycaemia may occur.
Pregnancy : Category C
In mice and rats L-asparaginase has been shown to retard the weight gain of mothers and foetuses when given in doses of more than 1000 IU/kg (the recommended human dose). Resorptions, gross abnormalities and skeletal abnormalities were observed. The intravenous administration of 50 or 100 IU/kg (one-twentieth or one-tenth of the human dose) to pregnant rabbits on Day 8 and 9 of gestation resulted in dose dependent embryotoxicity and gross abnormalities. There are no adequate and well-controlled studies in pregnant women. L-ASPERONE should be used during pregnancy only if the potential benefit justifies the potential risk to the foetus.
Nursing mothers :
It is not known whether this drug is secreted in human milk. Because many drugs are secreted in human milk and because of the potential for serious adverse reactions in nursing infants from L-ASPERONE, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Paediatric Use :
L-asparaginase toxicity is reported to be greater in adults than in paediatric patients.
Geriatric Use :
In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function and of concomittant disease or other drug therapy.
INTERACTIONS AND INCOMPATIBILITIES :
Tissue culture and animal studies indicate that L-asparaginase can diminish or abolish the effect of methotrexate on malignant cells. This effect on methotrexate activity persists as long as plasma asparagine levels are suppressed. These results would seem to dictate against the clinical use of methotrexate with L-asparaginase, or during the period following L-asparaginase therapy when plasma asparagine levels are below normal. Vincristine neurotoxicity may possibly be increased by use with intravenous L-asparaginase.
SIDE EFFECTS :
Hypersensitivity is the most frequent adverse reactions, such as urticaria, oedema of the larynx, bronchospasm, hypotension or even true anaphylactic shock. If these reactions occur, the treatment should be discontinued immediately and withdrawn.
• In paediatric patients with advanced leukaemia, a lower incidence of anaphylaxis has been reported with intramuscular administration, although there was a higher incidence of milder hypersensitivity reactions than with intravenous administration.
• Fatal hyperthermia has been reported.
Inhibition of protein synthesis :
• Clotting disorders including increased PT and thromboplastin time with hypofibrinogenaemia, decreased in anti-thrombin III, plasminogen and other factors (VII, IX, X and VIII), leading to possible bleeding and thrombotic complications.
• Decrease in serum insulin with hyperglycaemia; glucosuria and polyuria.
• Inhibition of lipoprotein lipase activity; increase in serum triglycerides and cholesterol.
• Decrease in brain concentrations of L-asparagine or L-glutamine, resulting in hyperammonaemia with clinical signs of metabolic encephalopathy such as consciousness disorders with confusion, stupor or coma in some patients. Some patients have shown central nervous system effects consisting of depression, somnolence, fatigue, coma, confusion, agitation and hallucinations varying from mild to severe. Rarely, a Parkinson-like syndrome has occurred, with tremor and a progressive increase in muscular tone. These side effects usually have reversed spontaneously after treatment was stopped. Therapy with asparaginase is associated with an increase in blood ammonia during the conversion of asparagine to aspartic acid by the enzyme. No clear correlation exists between the degree of
elevation of blood ammonia levels and the appearance of CNS changes.
INFORMATION FOR PATIENTS :
Serious Allergic Reactions :
Patients should be informed of the possibility of serious allergic reactions, including anaphylaxis, and advised to immediately report any swellings or difficulty breathing.
Thrombosis :
Patients should be advised to immediately report any severe headache. Arm or leg swelling, acute shortness of breath, and chest pain also should be reported immediately.
Pancreatitis :
Patients should be advised to immediately report any severe abdominal pain.
Glucose Intolerance :
Patients should be advised to report excessive thirst or any increase in the volume or frequency of urination.
OVERDOSAGE :
The acute intravenous LD50 of L-asparaginase for mice was about 5,00,000 IU/kg and for rabbits about 22,000 IU/kg.
PHARMACEUTICAL PRECAUTIONS :
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit. When reconstituted, L-ASPERONE should be a clear, colorless solution. If the solution becomes cloudy, discard.
STORAGE :
Store in a refrigerator between 2°C to 8°C (36°F to 46°F), protected from light.
Do not freeze.
SHELF LIFE :
24 months from the date of manufacture.
PRESENTATION :
L-ASPERONE contains 5,000 IU of lyophilized L-asparaginase.
L-ASPERONE contains 10,000 IU of lyophilized L-asparaginase.
Single vial pack.
Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.

 Cardiovascular
Cardiovascular