500 mg
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.
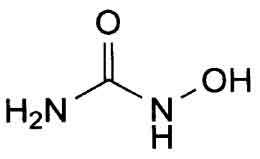
HYDROXYCARBAMIDE CAPSULES B.P. (Hydroxycarbamide) is an antineoplastic agent. Chemically, hydroxycarbamide is N-Hydroxyurea. The molecular formula is CH4N2O2 and molecular weight is 76.05.
STRUCTURAL FORMULA :
Its structural formula is :
HYDROXYCARBAMIDE CAPSULES B.P. is white to off-white crystalline powder, filled in hard gelatin capsule of size ‘0’.
COMPOSITION :
Each hard gelatin capsule contains :
Hydroxycarbamide B.P. 500 mg
Excipients q.s.
Approved colours used in empty capsule shells.
ACTIONS :
The precise mechanism by which hydroxycarbamide produces its cytotoxic effects cannot, at present be described, however, the reports of various studies in tissue culture in rats and man lend supports to the hypothesis that hydroxycarbamide causes an immediate inhibition of DNA synthesis without interfering with the synthesis of ribonucleic acid or of protein. This hypothesis explains why, under certain conditions, it may induce teratogenic effects. Three mechanisms of action have been postulated for the increased effectiveness of concomitant use of hydroxycarbamide therapy with irradiation on squamous cell (epidermoid) carcinomas of the head and neck. In vitro studies utilizing Chinese hamster cells suggest that hydroxycarbamide is lethal to normally radioresistants S - stage cells and holds other cells of the cell cycle in the G 1 or pre DNA synthesis stage where they are most susceptible to the effects of irradiation. The third mechanism of action has been theorized on the basis of in vitro studies of HeLa cells. It appears that hydroxycarbamide, by inhibition of DNA synthesis hinders the normal repair process of cell damaged but not killed by irradiation thereby decreasing their survival rate, RNA and protein synthesis have shown no alteration.
PHARMACOKINETICS :
After oral administration in man, hydroxycarbamide is readily absorbed from the gastrointestinal tract. The drug reaches peak serum concentrations within 2 hours and by 24 hours the concentration in the serum is essentially zero. Approximately 80 % of oral or intravenous dose of 7 mg/kg to 30 mg/kg may be recovered in the urine within 12 hours.
INDICATIONS :
Significant turnover response to HYDROXYCARBAMIDE CAPSULES B.P. has been demonstrated in melanoma, resistant chronic myelocytic leukaemia and recurrent metastatic or inoperable carcinoma of the ovary. HYDROXYCARBAMIDE CAPSULES B.P. used concomitantly with irradiation therapy is intended for use in the local control of primary squamous cell (epidermoid) carcinomas of the head and neck, excluding the lip and carcinoma of the cervix.
Administration :
HYDROXYCARBAMIDE CAPSULES B.P. is for oral administration.
Because of the rarity of melanoma, resistance chronic myelocytic leukaemia carcinoma of the ovary and carcinomas of head and neck in children, dosage regimens have not been established. All dosages should be based on the patients actual or ideal weight, whichever is less.Note : If the patient prefers or is unable to swallow capsules the contents of the capsules may be emptied into a glass of water and taken immediately. Some inert material used as a vehicle in the capsule may not dissolve and may float on the surface.
Dosage :
I. SOLID TUMOURS :
Intermittent Therapy :
80 mg/kg administered orally as a single dose every third day.
Continuous Therapy :
20 to 30 mg/kg administered orally as a single dose daily.
The intermittent dosage schedule offers the advantage of reduced toxicity since patients on this dosage regimen have rarely required complete discontinuance of therapy because of toxicity.
Concomitant Therapy with Irradiation :
(Carcinoma of the head and neck) 80 mg/kg administered orally as a single dose every third day. Administration of HYDROXYCARBAMIDE CAPSULES B.P. should be begun atleast seven days before initiation of irradiation and continued during radiotherapy as well as indefinitely afterwards provided that the patient may be kept under adequate observation and evidences no unusual or severe reactions. Irradiation should be given at the maximum dose considered appropriate for the particular therapeutic situation, adjustment of irradiation dosage is not usually necessary when HYDROXYCARBAMIDE CAPSULES B.P. is used concomitantly.
II. RESISTANT CHRONIC MYELOCYTIC LEUKAEMIA :
Until the intermittent therapy regimen has been evaluated continuous therapy (20 to 30 mg/kg administered orally as a single does daily) is recommended.An adequate trial period for determining the antineoplastic effectiveness for HYDROXYCARBAMIDE CAPSULES B.P. is 6 weeks therapy. When there is regression in tumour size or arrest in turnover growth, therapy should be continued indefinitely. Therapy should be interrupted if the white blood cell count drops below 2500/mm3 or the platelet count below 100,000/mm3. In these cases counts should be rechecked after 3 days, and therapy resumed when the counts rise significantly towards normal values. Since the haematopoietic rebound is prompt, it is usually necessary to omit only a few doses. If prompt rebound has not occurred during combined hydroxycarbamide and irradiation therapy, irradiation may also be interrupted. However, the need for postponement of irradiation therapy is rare, radiotherapy has usually been continued using the recommended dosage and technique. Anaemia, if it occurs, should be corrected with whole blood replacement, without interrupting HYDROXYCARBAMIDE CAPSULES B.P. therapy. Because haematopoiesis may be compromised by extensive irradiation or by other antineoplastic agents, it is recommended that HYDROXYCARBAMIDE CAPSULES B.P. be administered cautiously to patients who have recently received extensive radiation therapy or chemotherapy with other cytotoxic drugs.
Pain or discomfort from inflammation of the mucous membranes at the irradiated site (mucositis) is usually controlled by measures such as topical anaesthetics and orally administered analgesics. If the reaction is serve hydroxycarbamide therapy may be temporarily interrupted, if it is extremely severe, irradiation dosage may in addition, be temporarily postponed. However it has rarely been necessary to terminate these therapies. Severe gastric distress, such as nausea, vomiting and anorexia, resulting from combined therapy may usually be controlled by temporary interruption of HYDROXYCARBAMIDE CAPSULES B.P. administration, rarely has the additional interruption of irradiation been necessary.
CONTRAINDICATIONS :
HYDROXYCARBAMIDE CAPSULES B.P. is contraindicated in patients who have demonstrated a previous hypersensitivity to hydroxycarbamide or any other component of its formulations. HYDROXYCARBAMIDE CAPSULES B.P. is contraindicated in patients with marked bone marrow depression i.e. leucopenia (< 2500 WBC/mm3) or thrombocytopaenia (< 100,000 platelets/mm3), or severe anaemia.
WARNINGS :
Erythrocytic abnormalities : Megalobastic erythropoiesis which is self-limiting is often seen early in the course of Hydroxycarbamide therapy. The morphologic change resembles pernicious anaemia, but is not related to vitamin B or folic acid deficiency and reduce the rate of iron utilisation by erythrocytes but it does not appear to alter the red blood cell survival time.It should be used with caution in patients with marked renal dysfunction. Elderly patients may be more sensitive to the effect of this drug and may require a lower dose regimen.
The capsule shells contains “tartrazine” colour which may cause allergic reactions, including bronchial asthma, especially in patients who are allergic to the acetyl salicylic acid. Anaemia should be corrected before starting treatment. Secondarily to treatment may appear leukaemia and skin cancer. Preventive administration of folic acid is recommended.
PRECAUTIONS :
When appropriate, patients should be counselled concerning the use of contraceptive measures during therapy. Concurrent use of HYDROXYCARBAMIDE CAPSULES B.P. and other myelosuppressive agents or radiation therapy may increase the level of bone marrow depression or other adverse reactions. Patients should be informed to maintain adequate fluid intake and to consult the physician regarding missed doses. Therapy with hydroxycarbamide requires close supervision. The complete status of the blood including bone marrow examination, if indicated as well as kidney function and liver function should be determined prior to and repeatedly during treatment. Determination of haemoglobin level, total leukocyte counts and platelet counts should be performed atleast once a week throughout the course of hydroxycarbamide therapy. If the white blood cell count decreases to less than 2500/mm3 or the platelet count to less than 100,000/mm3 therapy should be interrupted until the values rise significantly towards normal levels. Anaemia if it occurs should be managed with whole blood replacement without interrupting hydroxycarbamide therapy.
Pregnancy : Category D
This drug affects DNA synthesis, hence may be potential mutagenic agent. The physician should carefully consider this possibility before administering this drug to female patients who may contemplate conception. Hydroxycarbamide is known to be teratogenic agent in
animals. Therefore it should not be used in woman who are or may become pregnant unless in the judgment of physician the potential benefits outweigh the possible hazards.
Nursing mothers :
HYDROXYCARBAMIDE CAPSULES B.P. is excreted in human milk. Because of the potential for serious adverse reactions in nursing infants from HYDROXYCARBAMIDE CAPSULES B.P., breastfeeding is not recommended during HYDROXYCARBAMIDE CAPSULES B.P. therapy.
Paediatric Use :
Safety and effectiveness in children have not been established.
INTERACTIONS AND INCOMPATIBILITIES :
Because of their effects on the gastro-intestinal mucosa antineoplastic have the potential to interfere with the absorption of other drugs given with the mouth. In addition many antineoplastics are inhibitors of certain subtypes of cytochrome P450 and some antineoplastics also metabolised by these enzymes, and in consequence the possibilities of interactions between antineoplastic, or between antineoplastics and concomitant medication, cannot be discounted. Drugs used in the treatment of gout. HIV patients treated with didanosine, stavudine or indinavir. Using live vaccine.
SIDE EFFECTS :
Adverse reactions have been primarily bone marrow depression (leucopenia, anaemia and occasionally thrombocytopaenia) and less frequently gastrointestinal symptoms (stomatitis, anorexia, nausea, vomiting, diarrhoea and constipation) and dermatological reactions such as maculopapular rash and facial erythema. Dysurea and alopecia occur very rarely. Large doses may produce moderate drowsiness. Neurological disturbances have occured extremely rarely and were limited to headache, dizziness, disorientation, hallucinations and convulsions. Hydroxycarbamide occasionally may cause temporary impairment of renal tubular function accompanied by elevation in serum uric acid, BUN and creatinine levels. Abnormal BSP retention has been reported. Fever, Chills malaise and elevation of hepatic enzymes have also been reported. Adverse reactions observed with combined hydroxycarbamide and irradiation therapy are similar to those reported with the use of hydroxycarbamide alone. These effects primarily include bone marrow depression (anaemia and leucopenia) and gastric irritation. Almost all patients receiving an adequate course of combined hydroxyurea and irradiation therapy will demonstrate concurrent leucopenia. Platelet depression (less than 100,000 cells/mm3) has occurred rarely and only in the presence of marked leucopenia. Gastric distress has also been reported with irradiation alone and in combination with hydroxycarbamide therapy. It should be borne in mind that therapeutic doses of irradiation alone produce the same adverse reaction as
hydroxycarbamide combined therapy may cause an increase in the incidence and severity of these side effects. Although inflammation of the mucous membranes at the irradiated site (mucositis) is attributed to irradiation alone, some investigators believe that the more severe cases are due to combination therapy. Fatal pancreatitis have been reported in patients with HIV using antiretroviral.
OVERDOSAGE AND TREATMENT OF OVERDOSAGE :
Immediate treatment consists of gastric lavage, followed by supportive therapy for cardiorespiratory systems if required. In the long term, careful monitoring is essential haematopoietic system and, if necessary, blood should be transfused. It has been reported mucocutaneous acute toxicity in patients receiving hydroxyurea at higher doses than recommended. Pain were observed, violet erythema, oedema on palms and soles followed by scaling of these, severe generalized hyperpigmentation of the skin, and severe acute stomatitis.
STORAGE :
Store below 30°C (86°F), protected from moisture and light.
Do not refrigerate.
SHELF LIFE :
24 months from the date of manufacture.
PRESENTATION :
HYDROXYCARBAMIDE CAPSULES B.P. contains Hydroxycarbamide B.P. 500 mg.
10 Strips of 10 Capsules per box.
Disclaimer : For the use of a Registered Medical Practitioner or a Hospital or a Institution only. Also it is not intended to be used by healthcare professionals or patients for the purpose of prescribing or administering these products. Questions regarding the complete and current content of product labeling / specification / presentation should be directed to SGPharma.

 Cardiovascular
Cardiovascular