2 mg
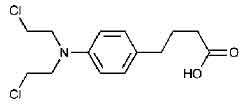
CHLORAMBUCIL TABLETS USP (Chlorambucil) is a bifunctional alkylating agent of the nitrogen mustard type that has been found active against selected human neoplastic diseases. Chemically, Chlorambucil is Benzenebutanoic acid, 4-[bis(2-chloroethyl)amino]-.The molecular formula is C14H19Cl2NO2 and molecular weight is 304.21.
STRUCTURAL FORMULA :
Its structural formula is :
CHLORAMBUCIL TABLETS USP are brown coloured round film coated tablet.
COMPOSITION :
Each film-coated tablet contains :
Chlorambucil USP 2 mg
Excipients q.s.
Colours : Titanium Dioxide B.P., Yellow Ferric Oxide NF, Red Ferric Oxide NF
ACTIONS :
Chlorambucil is a bifunctional alkylating agent of the nitrogen mustard type. Chlorambucil is cell cycle-phase nonspecific, although it is also cytotoxic to nonproliferating cells. Activity occurs as a result of formation of an unstable ethylenimmonium ion, which alkylates or binds with many intracellular molecular structures, including nucleic acids. Its cytotoxic action is primarily due to cross-linking of strands of DNA, which inhibits nucleic acid synthesis.
PHARMACOKINETICS :
Chlorambucil is rapidly and completely absorbed from the gastrointestinal tract. After single oral doses of 0.6 to 1.2 mg/kg, peak plasma chlorambucil levels (Cmax) are reached within 1 hour and the terminal elimination half-life (t1/2) of the parent drug is estimated at 1.5 hours. Chlorambucil undergoes rapid metabolism to phenylacetic acid mustard, the major metabolite, and the combined chlorambucil and phenylacetic acid mustard urinary excretion is extremely low-less than 1 % in 24 hours. In a study of 12 patients given single oral doses of 0.2 mg/kg of CHLORAMBUCIL TABLETS USP, the mean dose (12 mg) adjusted (±SD) plasma chlorambucil Cmax was 492 ± 160 ng/ml, the AUC was 883 ± 329 ng·h/ml, t1/2 was 1.3 ± 0.5 hours, and the tmax was 0.83 ± 0.53 hours. For the major metabolite, phenylacetic acid mustard, the mean dose (12 mg) adjusted (±SD) plasma Cmax was 306 ± 73 ng/ml, the AUC was 1204 ± 285 ng·h/ml, the t1/2 was 1.8 ± 0.4 hours, and the tmax was 1.9 ± 0.7 hours. Chlorambucil and its metabolites are extensively bound to plasma and tissue proteins. In vitro, chlorambucil is 99 % bound to plasma proteins, specifically albumin. Cerebrospinal fluid levels of chlorambucil have not been determined. Evidence of human teratogenicity suggests that the drug crosses the placenta. Chlorambucil is extensively metabolized in the liver primarily to phenylacetic acid mustard, which has antineoplastic activity. Chlorambucil and its major metabolite spontaneously degrade in vivo forming monohydroxy and dihydroxy derivatives. After a single dose of radiolabeled chlorambucil (14C), approximately 15 % to 60 % of the radioactivity appears in the urine after 24 hours. Again, less than 1 % of the urinary radioactivity is in the form of chlorambucil or phenylacetic acid mustard. In summary, the pharmacokinetic data suggest that oral chlorambucil undergoes rapid gastrointestinal absorption and plasma clearance and that it is almost completely metabolized, having extremely low urinary excretion.
INDICATIONS :
CHLORAMBUCIL TABLETS USP is indicated in the treatment of Hodgkin’s disease, certain forms of non-Hodgkin’s lymphoma, chronic lymphocytic leukaemia, and Waldenstrom’s macroglobulinaemia.
Administration :
For oral use.
Dosage :
Adults :
Hodgkin’s Disease : Used as a single agent in the palliative treatment of advanced disease a typical dosage is 0.2 mg/kg/day for 4 - 8 weeks. CHLORAMBUCIL TABLETS USP is usually included in combination therapy and a number of regimens have been used. CHLORAMBUCIL TABLETS USP has been used as an alternative to nitrogen mustard with a reduction in toxicity but similar therapeutic results. Non-Hodgkin’s Lymphoma : Used as a single agent the usual dosage is 0.1 - 0.2 mg/kg/day for 4 - 8 weeks initially, maintenance therapy is then given either by a reduced daily dosage or intermittent courses of treatment. CHLORAMBUCIL TABLETS USP is useful in the management of patients with advanced diffuse lymphocytic lymphoma and those who have relapsed after radiotherapy. There is no significant difference in the overall response rate obtained with chlorambucil as a single agent and combination chemotherapy in patients with advanced non-Hodgkin’s lympho-cytic lymphoma.
Chronic Lymphocytic Leukaemia : Treatment with CHLORAMBUCIL TABLETS USP is usually started after the patient has developed symptoms or when there is evidence of impaired bone marrow function (but not bone marrow failure) as indicated by the peripheral blood count. Initially CHLORAMBUCIL TABLETS USP is given at a dosage of 0.15 mg/kg/day until the total leucocyte count has fallen to 10,000 per μL. Treatment may be resumed 4 weeks after the end of the first course and continued at a dosage of 0.1 mg/kg/day. In a proportion of patients, usually after about 2 years of treatment, the blood leucocyte count is reduced to the normal range, enlarged spleen and lymph nodes become impalpable and the proportion of lymphocytes in the bone marrow is reduced to less than 20 per cent. Patients with evidence of bone marrow failure should first be treated with prednisolone and evidence of marrow regeneration should be obtained before commencing treatment with CHLORAMBUCIL TABLETS USP. Intermittent high dose therapy has been compared with
daily CHLORAMBUCIL TABLETS USP but no significant difference in therapeutic response or frequency of side effects was observed between the two treatment groups.
Waldenstrom’s Macroglobulinaemia : CHLORAMBUCIL TABLETS USP is the treatment of choice in this indication. Starting doses of 6 - 12 mg daily until leucopenia occurs are recommended followed by 2 - 8 mg daily indefinitely. Children : CHLORAMBUCIL TABLETS USP may be used in the management of Hodgkin’s disease and non-Hodgkin’s lymphomas in children. The dosage regimes are similar to those used in adults. Use in the Elderly : No specific studies have been carried out in the elderly, however, it may be advisable to monitor renal or hepatic function and if there is serious impairment then caution should be exercised.
CONTRAINDICATIONS :
CHLORAMBUCIL TABLETS USP should not be used in patients whose disease has demonstrated a prior resistance to the agent. Patients who have demonstrated hypersensitivity to chlorambucil should not be given the drug. There may be cross-hypersensitivity (skin rash) between chlorambucil and other alkylating agents.
WARNINGS :
Because of its carcinogenic properties, CHLORAMBUCIL TABLETS USP should not be given to patients with conditions other than chronic lymphatic leukaemia or malignant lymphomas. Convulsions, infertility, leukaemia, and secondary malignancies have been
observed when CHLORAMBUCIL TABLETS USP was employed in the therapy of malignant and non-malignant diseases. There are many reports of acute leukaemia arising in patients with both malignant and non-malignant diseases following chlorambucil treatment. In many instances, these patients also received other chemotherapeutic agents or some form of radiation therapy. The quantitation of the risk of chlorambucil-induction of leukaemia or carcinoma in humans is not possible. Evaluation of published reports of leukaemia developing in patients who have received chlorambucil (and other alkylating agents) suggests that the risk of leukaemogenesis increases with both chronicity of treatment and large cumulative doses. However, it has proved impossible to define a cumulative dose below which there is no risk of the induction of secondary malignancy. The potential benefits from chlorambucil therapy must be weighed on an individual basis against the possible risk of the induction of a secondary malignancy. CHLORAMBUCIL TABLETS USP has been shown to cause chromatid or chromosome damage in humans. Both reversible and permanent sterility have been observed in both sexes receiving chlorambucil. A high incidence of sterility has been documented when chlorambucil is administered to prepubertal and pubertal males. Prolonged or permanent azoospermia has also been observed in adult males. While most reports of gonadal dysfunction secondary to chlorambucil have related to males, the induction of amenorrhea in females with alkylating agents is well documented and chlorambucil is capable of producing amenorrhea. Autopsy studies of the ovaries from women with malignant lymphoma treated with combination chemotherapy including chlorambucil have shown varying degrees of fibrosis, vasculitis and depletion of primordial follicles. Rare instances of skin rash progressing to erythema multiforme, toxic epidermal necrolysis, or Stevens-Johnson syndrome have been reported. Chlorambucil should be discontinued promptly in patients who develop skin reactions.
PRECAUTIONS :
General :
Many patients develop a slowly progressive lymphopaenia during treatment. The lymphocyte count usually rapidly returns to normal levels upon completion of drug therapy. Most patients have some neutropaenia after the third week of treatment and this may continue for up to 10 days after the last dose. Subsequently, the neutrophil count usually rapidly returns to normal. Severe neutropaenia appears to be related to dosage and usually occurs only in patients who have received a total dosage of 6.5 mg/kg or more in one course of therapy with continuous dosing. About one quarter of all patients receiving the continuous-dose schedule, and one third of those receiving this dosage in 8 weeks or less may be expected to develop severe neutropaenia. While it is not necessary to discontinue chlorambucil at the first evidence of a fall in neutrophil count, it must be remembered that the fall may continue for 10 days after the last dose, and that as the total dose approaches 6.5 mg/kg, there is a risk of causing irreversible bone marrow damage. The dose of
chlorambucil should be decreased if leukocyte or platelet counts fall below normal values and should be discontinued for more severe depression.
Chlorambucil should not be given at full dosages before 4 weeks after a full course of radiation therapy or chemotherapy because of the vulnerability of the bone marrow to damage under these conditions. If the pretherapy leukocyte or platelet counts are depressed from
bone marrow disease process prior to institution of therapy, the treatment should be instituted at a reduced dosage. Persistently low neutrophil and platelet counts or peripheral lymphocytosis suggest bone marrow infiltration. If confirmed by bone marrow examination, the daily dosage of chlorambucil should not exceed 0.1 mg/kg. Chlorambucil appears to be relatively free from gastrointestinal side effects or other evidence of toxicity apart from the bone marrow depressant action. In humans, single oral doses of 20 mg or more may produce nausea and vomiting. Children with nephrotic syndrome and patients receiving high pulse doses of chlorambucil may have an increased risk of seizures. As with any potentially epileptogenic drug, caution should be exercised when administering chlorambucil to patients with a history of seizure disorder or head trauma, or who are receiving other potentially epileptogenic drugs.
Pregnancy : Pregnancy Category D
CHLORAMBUCIL TABLETS USP can cause foetal harm when administered to a pregnant woman. Unilateral renal agenesis has been observed in 2 offspring whose mothers received chlorambucil during the first trimester. Urogenital malformations, including absence of a kidney, were found in foetuses of rats given chlorambucil. There are no adequate and well-controlled studies in pregnant women. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the foetus. Women of childbearing potential should be advised to avoid becoming pregnant.
Nursing mothers :
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from chlorambucil, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Paediatric Use :
The safety and effectiveness in paediatric patients have not been established.
INTERACTIONS AND INCOMPATIBILITIES :
Vaccinations with live organism vaccines are not recommended in immunocompromised individuals. Patients receiving phenylbutazone may require a reduced dose of CHLORAMBUCIL TABLETS USP.
SIDE EFFECTS :
Haematologic : The most common side effect is bone marrow suppression. Although bone marrow suppression frequently occurs, it is usually reversible if the chlorambucil is withdrawn early enough. However, irreversible bone marrow failure has been reported.
Gastrointestinal : Gastrointestinal disturbances such as nausea and vomiting, diarrhoea, and oral ulceration occur infrequently.
CNS : Tremors, muscular twitching, myoclonia, confusion, agitation, ataxia, flaccid paresis, and hallucinations have been reported as rare adverse experiences to CHLORAMBUCIL TABLETS USP which resolve upon discontinuation of drug. Rare, focal and/or generalized seizures have been reported to occur in both children and adults at both therapeutic daily doses and pulse-dosing regimens and in acute overdose.
Dermatologic : Allergic reactions such as urticaria and angioneurotic oedema have been reported following initial or subsequent dosing. Skin hypersensitivity (including rare reports of skin rash progressing to erythema multiforme, toxic epidermal necrolysis, and Stevens-Johnson syndrome) has been reported.
Miscellaneous : Other reported adverse
reactions include : pulmonary fibrosis, hepatotoxicity and jaundice, drug fever, peripheral neuropathy, interstitial pneumonia, sterile cystitis, infertility, leukaemia, and secondary malignancies.
INFORMATION FOR PATIENTS :
Patients should be informed that the major toxicities of chlorambucil are related to hypersensitivity, drug fever, myelosuppression, hepatotoxicity, infertility, seizures, gastrointestinal toxicity, and secondary malignancies. Patients should never be allowed to take the drug without medical supervision and should consult their physician if they experience skin rash, bleeding, fever, jaundice, persistent cough, seizures, nausea, vomiting, amenorrhoea, or unusual lumps/masses. Women of childbearing potential should be advised to avoid becoming pregnant.
OVERDOSAGE :
Reversible pancytopaenia was the main finding of inadvertent overdoses of chlorambucil. Neurological toxicity ranging from agitated behavior and ataxia to multiple grand mal seizures has also occurred. As there is no known antidote, the blood picture should be closely monitored and general supportive measures should be instituted, together with appropriate blood transfusions, if necessary. Chlorambucil is not dialyzable. Oral LD50 single doses in mice are 123 mg/kg. In rats, a single intraperitoneal dose of 2.5 mg/kg of chlorambucil produces typical nitrogen-mustard effects; these include atrophy of the intestinal mucous membrane and lymphoid tissues, severe lymphopaenia becoming maximal in 4 days, anemia, and thrombocytopaenia. After this dose, the animals begin to recover within 1 - 3 days and appear normal in about a week, although the bone marrow may not become completely normal for about 3 weeks. An intraperitoneal dose of 18.5 mg/kg kills about 50 % of the rats with development of convulsions. As much as 50 mg/kg has been given orally to rats as a single dose, with recovery. Such a dose causes bradycardia, excessive salivation, haematuria, convulsions, and respiratory dysfunction.
TREATMENT OF OVERDOSAGE :
To enhance elimination - Immediate evacuation of the stomach.
Monitoring - Monitoring of blood counts at least three times a week for at least 3 weeks or until bone marrow function has recovered.
Supportive care - Supportive, symptomatic treatment. Patients in whom intentional overdose is confirmed or suspected should be referred for psychiatric consultation.
STORAGE :
Store in a refrigerator between 2°C to 8°C (36°F and 46°F).
Do not freeze.
SHELF LIFE :
24 months from the date of manufacture.
PRESENTATION :
CHLORAMBUCIL TABLETS USP contains Chlorambucil USP 2 mg.
One bottle of 25 tablets.
For the use of a Registered Medical Practitioner or a Hospital or a Institution only.

 Cardiovascular
Cardiovascular